
Alpha sheet
Encyclopedia
 |
 |
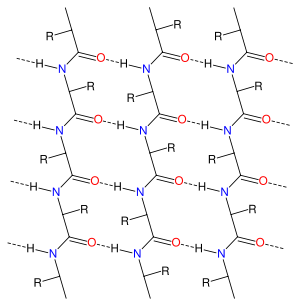
The alpha sheet (also known as an alpha pleated sheet or a polar pleated sheet) is a hypothetical secondary structure
Secondary structure
In biochemistry and structural biology, secondary structure is the general three-dimensional form of local segments of biopolymers such as proteins and nucleic acids...
in protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, first proposed by Linus Pauling
Linus Pauling
Linus Carl Pauling was an American chemist, biochemist, peace activist, author, and educator. He was one of the most influential chemists in history and ranks among the most important scientists of the 20th century...
and Robert Corey
Robert Corey
Robert Brainard Corey was an American biochemist, mostly known for his role in discovery of the α-helix and the β-sheet with Linus Pauling. Also working with Pauling was Herman Branson. Their discoveries were remarkably correct, with even the bond lengths being accurate until about 40 years later...
in 1951. The hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing pattern in an alpha sheet is similar to that of a beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
, but the orientation of the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
and amino groups in the peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
units is distinctive; in a single alpha strand, all of the carbonyl groups are oriented in the same direction on one side of the pleat, and all of the amino groups are oriented in the same direction on the opposite side of the sheet. Thus the alpha sheet accumulates an inherent separation of electrostatic charge, with one edge of the sheet exposing negatively charged carbonyl groups and the opposite edge exposing positively charged amino groups. Unlike the alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
and beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
, the alpha sheet configuration does not require all component amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues to lie within a single region of dihedral angle
Dihedral angle
In geometry, a dihedral or torsion angle is the angle between two planes.The dihedral angle of two planes can be seen by looking at the planes "edge on", i.e., along their line of intersection...
s; instead, the alpha sheet contains residues of alternating dihedrals in the traditional right-handed (αR) and left-handed (αL) helical regions of Ramachandran space
Ramachandran plot
-Introduction and early history:A Ramachandran plot , originally developed in 1963 by G. N. Ramachandran C. Ramakrishnan and V...
. Although the alpha sheet is only rarely observed in natural protein structure
Protein structure
Proteins are an important class of biological macromolecules present in all organisms. Proteins are polymers of amino acids. Classified by their physical size, proteins are nanoparticles . Each protein polymer – also known as a polypeptide – consists of a sequence formed from 20 possible L-α-amino...
s, it has been speculated to play a role in amyloid disease and it was found to be a stable form for amyloid
Amyloid
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
ogenic proteins in one set of molecular dynamics
Molecular dynamics
Molecular dynamics is a computer simulation of physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a period of time, giving a view of the motion of the atoms...
simulations. Alpha sheets have also been observed in X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
structures of designed peptides.
Experimental evidence
When Pauling and Corey first proposed the alpha sheet, they suggested that it agreed well with fiber diffractionFiber diffraction
Fiber diffraction is a subarea of scattering, an area in which molecular structure is determined from scattering data . In fiber diffraction the scattering pattern does not change, as the sample is rotated about a unique axis...
results from beta-keratin
Beta-keratin
β-keratin or beta-keratin is rich in stacked β pleated sheets, in contrast to alpha-keratin, a fibrous protein rich in alpha helices.β-keratin is found in reptiles...
fibers. However, since the alpha sheet did not appear to be energetically favorable, they argued that beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
s would occur more commonly among normal proteins, and subsequent demonstration that beta-keratin is made of beta sheets consigned the alpha sheet proposal to obscurity. Recently the alpha strand conformation has been observed in isolated instances in native state
Native state
In biochemistry, the native state of a protein is its operative or functional form. While all protein molecules begin as simple unbranched chains of amino acids, once completed they assume highly specific three-dimensional shapes; that ultimate shape, known as tertiary structure, is the folded...
proteins as solved by X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
or protein NMR, although an extended alpha sheet was not identified in any known natural protein. Native proteins identified as containing alpha-strand regions or alpha-sheet-patterned hydrogen bonding include synaptotagmin
Synaptotagmin
Synaptotagmins constitute a family of membrane-trafficking proteins that are characterized by an N-terminal transmembrane region , a variable linker, and two C-terminal C2 domains - C2A and C2B. There are 15 members in the mammalian synaptotagmin family...
, lysozyme
Lysozyme
Lysozyme, also known as muramidase or N-acetylmuramide glycanhydrolase, are glycoside hydrolases, enzymes that damage bacterial cell walls by catalyzing hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in a peptidoglycan and between...
, and potassium channel
Potassium channel
In the field of cell biology, potassium channels are the most widely distributed type of ion channel and are found in virtually all living organisms. They form potassium-selective pores that span cell membranes...
s, where the alpha-strands line the ion-conducting pore.
Alpha-sheet conformations have been observed in crystal structures of short non-natural peptides, especially those containing a mixture of L and D amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s. The first crystal structure containing an alpha sheet was observed in the capped tripeptide Boc
Di-tert-butyl dicarbonate
Di-tert-butyl dicarbonate is a reagent widely used in organic synthesis. This carbonate ester reacts with amines to give N-tert-butoxycarbonyl or so-called t-BOC derivatives. These derivatives do not behave as amines, which allows certain subsequent transformations to occur that would have...
–Ala
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
L–a
Epimer
In chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
-Ile
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
D–Ile
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
L–O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
Me. Other peptides that assume alpha-sheet structures include capped diphenyl-glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
-based dipeptides and tripeptides.
Role in amyloidogenesis
The alpha sheet has been proposed as a possible intermediate state in the conformational changeConformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
in the formation of amyloid
Amyloid
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. Abnormal accumulation of amyloid in organs may lead to amyloidosis, and may play a role in various neurodegenerative diseases.-Definition:...
fibrils by peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
s and proteins such as amyloid beta
Amyloid beta
Amyloid beta is a peptide of 36–43 amino acids that is processed from the Amyloid precursor protein. While it is most commonly known in association with Alzheimer's disease, it does not exist specifically to cause disease...
, poly-glutamine repeats
Polyglutamine tract
A polyglutamine tract or polyQ tract is a portion of a protein consisting of a sequence of several glutamine units. A tract typically consists of about 10 to a few hundred such units....
, lysozyme
Lysozyme
Lysozyme, also known as muramidase or N-acetylmuramide glycanhydrolase, are glycoside hydrolases, enzymes that damage bacterial cell walls by catalyzing hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in a peptidoglycan and between...
, prion
Prion
A prion is an infectious agent composed of protein in a misfolded form. This is in contrast to all other known infectious agents which must contain nucleic acids . The word prion, coined in 1982 by Stanley B. Prusiner, is a portmanteau derived from the words protein and infection...
proteins, and transthyretin
Transthyretin
Transthyretin is a serum and cerebrospinal fluid carrier of the thyroid hormone thyroxine and retinol binding protein bound to retinol. This is how transthyretin gained its name, transports thyroxine and retinol...
repeats, all of which are associated with protein misfolding
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
disease. For example, amyloid beta is a major component of amyloid plaques in the brains of Alzheimer's disease
Alzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
patients, and polyglutamine repeats in the huntingtin
Huntingtin
The Huntingtin gene, also called HTT or HD gene, is the IT15 gene which codes for a protein called the huntingtin protein...
protein are associated with Huntington's disease
Huntington's disease
Huntington's disease, chorea, or disorder , is a neurodegenerative genetic disorder that affects muscle coordination and leads to cognitive decline and dementia. It typically becomes noticeable in middle age. HD is the most common genetic cause of abnormal involuntary writhing movements called chorea...
. These proteins undergo a conformational change
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
from largely random coil
Random coil
A random coil is a polymer conformation where the monomer subunits are oriented randomly while still being bonded to adjacent units. It is not one specific shape, but a statistical distribution of shapes for all the chains in a population of macromolecules...
or alpha helix
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
structures to the highly ordered beta sheet
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
structures found in amyloid fibrils. Most beta sheets in known proteins are "twisted" about 15° for optimal hydrogen bonding and steric packing; however, some evidence from electron crystallography
Electron crystallography
Electron crystallography is a method to determine the arrangement of atoms in solids using a transmission electron microscope .- Comparison with X-ray crystallography :...
suggests that at least some amyloid fibrils contain "flat" sheets with only 1-2.5° of twist. An alpha-sheet amyloid intermediate is suggested to explain some anomalous features of the amyloid fibrillization process, such as the evident amino acid sequence dependence of amyloidogenesis despite the belief that the amyloid fold is mainly stabilized by the protein backbone and the acceleration of fibrillization in the presence of a magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
.
Xu, using atomic force microscopy, has shown that formation of amyloid fibers is a two-step process in which proteins first aggregate into colloidal spheres of ~20 nm diameter. The spheres then join together spontaneously to form linear chains, which evolve into mature amyloid fibers. The formation of these linear chains appears to be driven by the development of an electrostatic dipole in each of the colloidal spheres strong enough to overcome coulomb repulsion. This suggests a possible mechanism by which alpha sheet may promote amyloid aggregation; the peptide bond has a relatively large intrinsic electrostatic dipole, but normally the dipoles of nearby bonds cancel each other out. In the alpha sheet, unlike other conformations, the peptide bonds are oriented in parallel so that the dipoles of the individual bonds can add up to create a strong overall electrostatic dipole.
Notably, the protein lysozyme
Lysozyme
Lysozyme, also known as muramidase or N-acetylmuramide glycanhydrolase, are glycoside hydrolases, enzymes that damage bacterial cell walls by catalyzing hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in a peptidoglycan and between...
is among the few native-state proteins shown to contain an alpha-strand region; lysozyme from both chicken
Chicken
The chicken is a domesticated fowl, a subspecies of the Red Junglefowl. As one of the most common and widespread domestic animals, and with a population of more than 24 billion in 2003, there are more chickens in the world than any other species of bird...
s and human
Human
Humans are the only living species in the Homo genus...
s contains an alpha strand located close to the site of a mutation
Mutation
In molecular biology and genetics, mutations are changes in a genomic sequence: the DNA sequence of a cell's genome or the DNA or RNA sequence of a virus. They can be defined as sudden and spontaneous changes in the cell. Mutations are caused by radiation, viruses, transposons and mutagenic...
known to cause hereditary amyloidosis in humans, usually an autosomal dominant genetic disease. Molecular dynamics simulations of the mutant protein reveal that the region around the mutation assumes an alpha strand conformation. Lysozyme is among the naturally occurring proteins known to form amyloid fibers under experimental conditions, and both natively alpha-strand region and the mutation site fall within the larger region identified as the core of lysozyme amyloid fibrillogenesis.
A mechanism for direct alpha sheet and beta sheet interconversion has also been suggested, based on peptide plane flipping
Peptide plane flipping
Peptide plane flipping is a type of conformational change that can occur in proteins by which the dihedral angles of adjacent amino acids undergo large-scale rotations with little displacement of the side chains...
in which the αRαL dipeptide inverts to produce a ββ dihedral angle conformation. This process has also been observed in simulations of transthyretin and implicated as occurring naturally in certain protein families
Protein family
A protein family is a group of evolutionarily-related proteins, and is often nearly synonymous with gene family. The term protein family should not be confused with family as it is used in taxonomy....
by examination of their dihedral angle conformations in crystal structures.

