Aluminium isopropoxide
Encyclopedia
Aluminium isopropoxide is the chemical compound
usually described with the formula Al(O-i-Pr)3, where i-Pr is the isopropyl
group (CH(CH3)2). This colourless solid is a useful reagent
in organic synthesis
. The structure of this compound is complex, possibly time-dependent, and may depend on solvent.
and X-ray crystallography
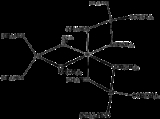
. The species is described by the formula Al(μ-O-i-Pr)2Al(O-i-Pr)23. The unique central Al is octahedral surrounded by three bidentate "Al(O-i-Pr)4-" ligand
s, each featuring tetrahedral Al. The idealised point group symmetry
is D3. The tert-butoxide is a dimer with the formula Al2(μ-O-t-Bu)2(O-t-Bu)4 It is prepared analogously to the isopropoxide.
, and 5 g of mercuric chloride at reflux. The process occurs via the formation of an amalgam
of the aluminium. A catalytic amount of iodine is sometimes added to initiate the reaction, which can be quite vigorous. Young et al. achieved an 85–90% yield, after purification by distillation at 140–150 °C (5 mm Hg).
s. In these reactions, it is assumed that the tetrameric cluster disagregates.
Being a basic alkoxide
, Al(O-i-Pr)3 has been also investigated as a catalyst for ring opening polymerization of cyclic esters.
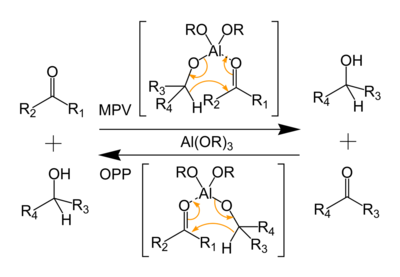
(catalytic transformation of aldehydes into esters). It was later found also to display catalytic activity as a reducing agent by Meerwein
and Schmidt in the Meerwein-Ponndorf-Verley reduction
("MPV") in 1925. The reverse of the MPV reaction, oxidation of an alcohol to a ketone, is termed the Oppenauer oxidation
. The original Oppenauer oxidation employed aluminium butoxide in place of the isoproxide.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
usually described with the formula Al(O-i-Pr)3, where i-Pr is the isopropyl
Isopropyl
In organic chemistry, isopropyl is a propyl with a group attached to the secondary carbon. If viewed as a functional group an isopropyl is an organic compound with a propyl group attached at its secondary carbon.The bond is therefore on the middle carbon....
group (CH(CH3)2). This colourless solid is a useful reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. The structure of this compound is complex, possibly time-dependent, and may depend on solvent.
Structure
The structure of the metal alkoxides are often complex and aluminium isopropoxide is no exception. The complexity is also reflected in the disputed melting point for the material which could reflect the presence of trace impurities, such as water, slow oligomerisation ("aging") or both. For aluminium isopropoxide this phenomenon is mainly due to the trimer-tetramer transformation described in detail in the early works by Turova et al. The tetrameric structure of the solid crystalline material was verified by NMR spectroscopyNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
and X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
. The species is described by the formula Al(μ-O-i-Pr)2Al(O-i-Pr)23. The unique central Al is octahedral surrounded by three bidentate "Al(O-i-Pr)4-" ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s, each featuring tetrahedral Al. The idealised point group symmetry
Symmetry group
The symmetry group of an object is the group of all isometries under which it is invariant with composition as the operation...
is D3. The tert-butoxide is a dimer with the formula Al2(μ-O-t-Bu)2(O-t-Bu)4 It is prepared analogously to the isopropoxide.
Preparation
A widely accepted method for preparing aluminium isopropoxide was published in 1936 by Young, Hartung, and Crossley. Their procedure entails heating a mixture of 100 g of aluminium, 1200 mL of isopropyl alcoholIsopropyl alcohol
Isopropyl alcohol is a common name for a chemical compound with the molecular formula C3H8O. It is a colorless, flammable chemical compound with a strong odor...
, and 5 g of mercuric chloride at reflux. The process occurs via the formation of an amalgam
Amalgam (chemistry)
An amalgam is a substance formed by the reaction of mercury with another metal. Almost all metals can form amalgams with mercury, notable exceptions being iron and platinum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore.The...
of the aluminium. A catalytic amount of iodine is sometimes added to initiate the reaction, which can be quite vigorous. Young et al. achieved an 85–90% yield, after purification by distillation at 140–150 °C (5 mm Hg).
Reactions
In a MPV reduction, ketones and aldehydes are reduced to alcohols concomitant with the formation of acetone. This reduction relies on an equilibrium process, hence it produces the thermodynamic product. Conversely, in the Oppenauer Oxidation, secondary alcohols are converted to ketones, and homoallylic alcohols are converted to α,β-unsaturated carbonylCarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
s. In these reactions, it is assumed that the tetrameric cluster disagregates.

Being a basic alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
, Al(O-i-Pr)3 has been also investigated as a catalyst for ring opening polymerization of cyclic esters.
History
Aluminium isopropoxide was first reported in the Dissertation of Alexandre Tischenko in the Annals of the Russian Physico-Chemical society in 1898. This contribution included detailed description of its synthesis, peculiar physico-chemical behavior and catalytic activity in Tishchenko reactionTishchenko reaction
The Tishchenko reaction is a chemical reaction that involves disproportionation of an aldehyde lacking a hydrogen atom in the alpha position in the presence of an alkoxide. The reaction product is an ester. Catalysts are aluminium alkoxides or sodium alkoxides...
(catalytic transformation of aldehydes into esters). It was later found also to display catalytic activity as a reducing agent by Meerwein
Hans Meerwein
Hans Meerwein was a German chemist.His name is present in the names of several reactions and reagents, for example the Meerwein-Ponndorf-Verley reduction, the Wagner-Meerwein rearrangement...
and Schmidt in the Meerwein-Ponndorf-Verley reduction
Meerwein-Ponndorf-Verley reduction
The Meerwein-Ponndorf-Verley Reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminumalkoxide catalysis in the presence of a sacrificial alcohol...
("MPV") in 1925. The reverse of the MPV reaction, oxidation of an alcohol to a ketone, is termed the Oppenauer oxidation
Oppenauer oxidation
Oppenauer oxidation, named after Rupert Viktor Oppenauer, is a gentle method for selectively oxidizing secondary alcohols to ketones.The reaction is the opposite of Meerwein-Ponndorf-Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess acetone...
. The original Oppenauer oxidation employed aluminium butoxide in place of the isoproxide.

