Anthracene
Encyclopedia
Anthracene is a solid polycyclic aromatic hydrocarbon
consisting of three fused benzene
rings. It is a component of coal-tar. Anthracene is used in the production of the red
dye
alizarin
and other dyes. Anthracene is colorless but exhibits a blue (400-500 nm peak) fluorescence
under ultraviolet
light.
In 2010, a strong absorption band
of anthracene was observed along a line of sight to a star in the open cluster
IC 348, and this may be associated with an intervening molecular cloud
.
and carbazole
. A classic laboratory method for the preparation of anthracene is by cyclodehydration of o-methyl- or o-methylene-substituted diarylketones in the so-called Elbs reaction
.
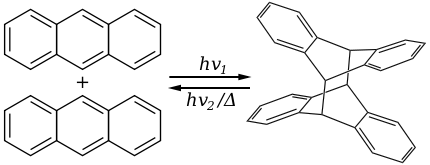
s by the action of UV light:
The dimer is connected by a pair of new carbon-carbon bonds, the result of the [4+4] cycloaddition
. The dimer reverts to anthracene thermally or with UV irradiation below 300 nm. The reversible dimerization and the photochromic properties of anthracenes are the basis of potential applications. Substituted anthracene derivatives behave similarly. The reaction is affected by the presence of oxygen
.
In general, reduction of anthracene yields 9,10-dihydroanthracene (destroying the aromaticity of the center ring) rather than 1,4-dihydroanthracene (which would destroy the aromaticity of one of the terminal rings). This preference for reduction at the 9 and 10 positions is explained by the fact that aromatic stabilization energy is directly correlated with the number of conjugated pi bonds in an aromatic system. Since 9,10-dihydroanthracene in essence preserves two "benzene" rings (a total of 6 conjugated pi bonds), whereas the 1,4-isomer preserves only one and a half such rings (a total of 5 pi bonds); the latter is not the thermodynamically favorable product. Likewise, electrophilic substitution
occurs at the "9" and "10" positions of the center ring.
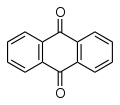
Oxidation occurs readily, giving anthraquinone
, C14H8O2 (below), for example using hydrogen peroxide
and VO(acac)2.
Anthracene is an organic semiconductor
. It is used as a scintillator
for detectors of high energy photon
s, electron
s and alpha particle
s. Plastics, such as polyvinyltoluene, can be doped with anthracene to produce a plastic scintillator that is approximately water-equivalent for use in radiation therapy
dosimetry
. Anthracene's emission spectrum
peaks at between 400 nm and 440 nm.
It is also used in wood
preservative
s, insecticide
s, and coating materials
.
are 1-hydroxyanthracene and 2-hydroxyanthracene, homologous to phenol
and naphthol
s, and hydroxyanthracene (also called anthrol, and anthracenol) are pharmacologically
active.
Anthracene may also be found with multiple hydroxyl groups, as in 9,10-dihydroxyanthracene
.
), anthracene is not carcinogenic but has been recently included in the Substances of Very High Concern list (SVHC) by the European Chemicals Agency (ECHA) http://echa.europa.eu/chem_data/candidate_list_table_en.asp because it is considered Persistent, Bioaccumulative and Toxic (PBT) for freshwater and marine ecosystems http://echa.europa.eu/doc/candidate_list/svhc_supdoc_anthracene_publication.pdf within the REACH framework. Anthracene, as many other PAHs, is generated during combustion processes: Exposure to humans happens mainly through tobacco smoke and ingestion of food contaminated with combustion products http://www.cie.iarc.fr/htdocs/monographs/vol32/anthracene.html.
Polycyclic aromatic hydrocarbon
Polycyclic aromatic hydrocarbons , also known as poly-aromatic hydrocarbons or polynuclear aromatic hydrocarbons, are potent atmospheric pollutants that consist of fused aromatic rings and do not contain heteroatoms or carry substituents. Naphthalene is the simplest example of a PAH...
consisting of three fused benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
rings. It is a component of coal-tar. Anthracene is used in the production of the red
Red
Red is any of a number of similar colors evoked by light consisting predominantly of the longest wavelengths of light discernible by the human eye, in the wavelength range of roughly 630–740 nm. Longer wavelengths than this are called infrared , and cannot be seen by the naked eye...
dye
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
alizarin
Alizarin
Alizarin or 1,2-dihydroxyanthraquinone is an organic compound with formula that has been used throughout history as a prominent dye, originally derived from the roots of plants of the madder genus.Alizarin was used as a red dye for the English parliamentary "new model" army...
and other dyes. Anthracene is colorless but exhibits a blue (400-500 nm peak) fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
under ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
light.
In 2010, a strong absorption band
Absorption band
An absorption band is a range of wavelengths, frequencies or energies in the electromagnetic spectrum which are able to excite a particular transition in a substance...
of anthracene was observed along a line of sight to a star in the open cluster
Open cluster
An open cluster is a group of up to a few thousand stars that were formed from the same giant molecular cloud and have roughly the same age. More than 1,100 open clusters have been discovered within the Milky Way Galaxy, and many more are thought to exist...
IC 348, and this may be associated with an intervening molecular cloud
Molecular cloud
A molecular cloud, sometimes called a stellar nursery if star formation is occurring within, is a type of interstellar cloud whose density and size permits the formation of molecules, most commonly molecular hydrogen ....
.
Production
Commercial anthracene is obtained from coal tar, common impurities being phenanthrenePhenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbon composed of three fused benzene rings. The name phenanthrene is a composite of phenyl and anthracene. In its pure form, it is found in cigarette smoke and is a known irritant, photosensitizing skin to light...
and carbazole
Carbazole
Carbazole is an aromatic heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene ring fused on either side of a five-membered nitrogen-containing ring...
. A classic laboratory method for the preparation of anthracene is by cyclodehydration of o-methyl- or o-methylene-substituted diarylketones in the so-called Elbs reaction
Elbs reaction
The Elbs reaction is an organic reaction describing the pyrolysis of an ortho methyl substituted benzophenone to condensed polyaromatic. The reaction is named after its inventor, the German chemist Karl Elbs also responsible for the Elbs oxidation...
.
Reactions
Anthracene photodimerizePhotochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
s by the action of UV light:
The dimer is connected by a pair of new carbon-carbon bonds, the result of the [4+4] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
. The dimer reverts to anthracene thermally or with UV irradiation below 300 nm. The reversible dimerization and the photochromic properties of anthracenes are the basis of potential applications. Substituted anthracene derivatives behave similarly. The reaction is affected by the presence of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
.
In general, reduction of anthracene yields 9,10-dihydroanthracene (destroying the aromaticity of the center ring) rather than 1,4-dihydroanthracene (which would destroy the aromaticity of one of the terminal rings). This preference for reduction at the 9 and 10 positions is explained by the fact that aromatic stabilization energy is directly correlated with the number of conjugated pi bonds in an aromatic system. Since 9,10-dihydroanthracene in essence preserves two "benzene" rings (a total of 6 conjugated pi bonds), whereas the 1,4-isomer preserves only one and a half such rings (a total of 5 pi bonds); the latter is not the thermodynamically favorable product. Likewise, electrophilic substitution
Electrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a group in a compound, typically but not always hydrogen. Electrophilic aromatic substitution is characteristic of aromatic compounds and is an important way of introducing functional groups onto benzene...
occurs at the "9" and "10" positions of the center ring.
Oxidation occurs readily, giving anthraquinone
Anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene is an aromatic organic compound with formula . Several isomers are possible, each of which can be viewed as a quinone derivative...
, C14H8O2 (below), for example using hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
and VO(acac)2.
Uses
Anthracene is converted mainly to anthroquinone, a precursor to dyes.Anthracene is an organic semiconductor
Organic semiconductor
An organic semiconductor is an organic material with semiconductor properties. Single molecules, short chain and organic polymers can be semiconductive. Semiconducting small molecules include the polycyclic aromatic compounds pentacene, anthracene, and rubrene...
. It is used as a scintillator
Scintillator
A scintillator is a special material, which exhibits scintillation—the property of luminescence when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate, i.e., reemit the absorbed energy in the form of light...
for detectors of high energy photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s, electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s and alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
s. Plastics, such as polyvinyltoluene, can be doped with anthracene to produce a plastic scintillator that is approximately water-equivalent for use in radiation therapy
Radiation therapy
Radiation therapy , radiation oncology, or radiotherapy , sometimes abbreviated to XRT or DXT, is the medical use of ionizing radiation, generally as part of cancer treatment to control malignant cells.Radiation therapy is commonly applied to the cancerous tumor because of its ability to control...
dosimetry
Dosimetry
Radiation dosimetry is the measurement and calculation of the absorbed dose in matter and tissue resulting from the exposure to indirect and direct ionizing radiation...
. Anthracene's emission spectrum
Emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted by the element's atoms or the compound's molecules when they are returned to a lower energy state....
peaks at between 400 nm and 440 nm.
It is also used in wood
Wood
Wood is a hard, fibrous tissue found in many trees. It has been used for hundreds of thousands of years for both fuel and as a construction material. It is an organic material, a natural composite of cellulose fibers embedded in a matrix of lignin which resists compression...
preservative
Preservative
A preservative is a naturally occurring or synthetically produced substance that is added to products such as foods, pharmaceuticals, paints, biological samples, wood, etc. to prevent decomposition by microbial growth or by undesirable chemical changes....
s, insecticide
Insecticide
An insecticide is a pesticide used against insects. They include ovicides and larvicides used against the eggs and larvae of insects respectively. Insecticides are used in agriculture, medicine, industry and the household. The use of insecticides is believed to be one of the major factors behind...
s, and coating materials
Commodity
In economics, a commodity is the generic term for any marketable item produced to satisfy wants or needs. Economic commodities comprise goods and services....
.
Derivatives
A variety of anthracene derivatives find niche uses. Derivatives having a hydroxyl groupHydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
are 1-hydroxyanthracene and 2-hydroxyanthracene, homologous to phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
and naphthol
Naphthol
Naphthol may refer to:* 1-Naphthol* 2-Naphthol...
s, and hydroxyanthracene (also called anthrol, and anthracenol) are pharmacologically
Pharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
active.
Anthracene may also be found with multiple hydroxyl groups, as in 9,10-dihydroxyanthracene
9,10-Dihydroxyanthracene
9,10-Dihydroxyanthracene is the hydroquinone form of 9,10-anthraquinone and is formed when AQ is used as a redox catalyst in various industrial processes...
.
Toxicology
Unlike other polycyclic aromatic hydrocarbons (PAHPolycyclic aromatic hydrocarbon
Polycyclic aromatic hydrocarbons , also known as poly-aromatic hydrocarbons or polynuclear aromatic hydrocarbons, are potent atmospheric pollutants that consist of fused aromatic rings and do not contain heteroatoms or carry substituents. Naphthalene is the simplest example of a PAH...
), anthracene is not carcinogenic but has been recently included in the Substances of Very High Concern list (SVHC) by the European Chemicals Agency (ECHA) http://echa.europa.eu/chem_data/candidate_list_table_en.asp because it is considered Persistent, Bioaccumulative and Toxic (PBT) for freshwater and marine ecosystems http://echa.europa.eu/doc/candidate_list/svhc_supdoc_anthracene_publication.pdf within the REACH framework. Anthracene, as many other PAHs, is generated during combustion processes: Exposure to humans happens mainly through tobacco smoke and ingestion of food contaminated with combustion products http://www.cie.iarc.fr/htdocs/monographs/vol32/anthracene.html.
See also
- PhenanthrenePhenanthrenePhenanthrene is a polycyclic aromatic hydrocarbon composed of three fused benzene rings. The name phenanthrene is a composite of phenyl and anthracene. In its pure form, it is found in cigarette smoke and is a known irritant, photosensitizing skin to light...
- TetraceneTetraceneTetracene, also called naphthacene, is a polycyclic aromatic hydrocarbon. It has the appearance of a pale orange powder. Tetracene is the four-ringed member of the series of acenes, the previous one being anthracene and the next one being pentacene.Tetracene is a molecular organic semiconductor,...
- 9,10-Dithioanthracene9,10-Dithioanthracene9,10-Dithioanthracene is the first molecule ever to be able to "walk" in a straight line by, in effect, mimicking the bipedal motion of a human being. It is an organic molecule composed of a coal tar derivative called anthracene linked to a pair of sulfur-bearing functional groups on either side ,...
, which migrates in a straight line when adsorbed on a flat copper surface.