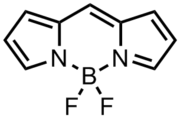
BODIPY
Encyclopedia
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
dyes. It is composed of dipyrromethene complexed with a disubstituted boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
atom, typically a BF2 unit. The IUPAC name for the BODIPY core is 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene. Due to instability of the required unsubstituted dipyrromethene precursor, the unsubstituted BODIPY dye had not been prepared until 2009. Three independent groups reported different syntheses of the title compound.
Properties
BODIPY dyes are notable for their uniquely small Stokes shiftStokes shift
Stokes shift is the difference between positions of the band maxima of the absorption and emission spectra of the same electronic transition. It is named after Irish physicist George G. Stokes. When a system absorbs a photon, it gains energy and enters an excited state...
, high, environment-independent fluorescence quantum yield
Quantum yield
The quantum yield of a radiation-induced process is the number of times that a defined event occurs per photon absorbed by the system. The "event" may represent a chemical reaction, for example the decomposition of a reactant molecule:...
s, often approaching 100% even in water, and sharp excitation and emission peaks contributing to overall brightness. The combination of these qualities makes BODIPY fluorophore an important tool in a variety of imaging applications. The position of the absorption and emission bands remain almost unchanged in solvents of different polarity as the dipole moment
Polarity
In physics, polarity is a description of an attribute, typically a binary attribute , or a vector . For example:* An electric charge has a polarity of either positive or negative....
and transition dipole are orthogonal to each other.
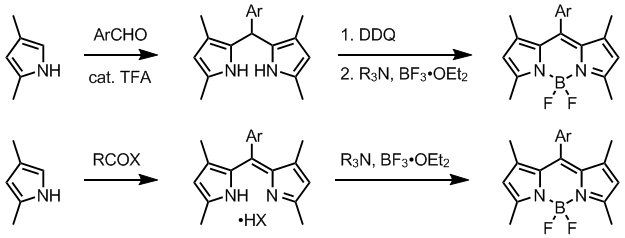
Synthesis
BODIPY are prepared by reacting a dipyrromethene precursor with boron trifluorideBoron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
etherate in the presence of a tertiary amine. Dipyrromethenes are accessed from a suitable pyrrole, by several methods. Normally, one alpha-position in employed pyrroles is substituted and the other is free. Condensation of such pyrrole, often available from Knorr pyrrole synthesis
Knorr pyrrole synthesis
The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles '. The method involves the reaction of an α-amino-ketone ' and a compound containing a methylene group α- to a carbonyl group '.-Method:The mechanism requires zinc and acetic acid as catalysts...
, with an aromatic aldehyde in the presence of TFA
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
gives dipyrromethane, which is oxidized to dipyrromethene using a quinone oxidant such as DDQ
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone is the chemical reagent with formula C8Cl2N2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols and steroid ketones in organic chemistry. DDQ decomposes in water, but is stable in aqueous mineral acid.-Preparation:Synthesis of DDQ...
or p-chloranil.
Alternatively, dipyrromethenes are prepared directly by reacting a pyrrole with an activated carboxylic acid derivative, usually acid chloride. Unsymmetrical dipyrromethenes can be obtained by condensing pyrroles with 2-acylpyrroles. Intermediate dipyrromethanes may be isolated and purified, but isolation of dipyrromethenes is usually compromised by their instability.