Barrelene
Encyclopedia
Barrelene is a bicyclic organic compound
with chemical formula
C8H8 and systematic name
bicyclo[2.2.2]octa-2,5,7-triene. First synthesized and described by H. E. Zimmerman
in 1960 the name derives from the obvious resemblance with a barrel
, with the staves being three ethylene
units attached to two methine
groups. It is the formal Diels-Alder adduct of benzene
and acetylene
. Due to its unusual molecular geometry
the compound is of considerable interest to theoretical chemists. Like benzene
, barrelene has a set of 6 cyclic, but not planar, overlapping p-orbitals. Because it is not possible to avoid a destabilizing overlap of opposite-sign lobes, the structure represents Möbius aromaticity
.
Triptycene
s, with the alkene groups part of an arene, are related compounds. It is also a starting material for many other organic compounds such as semibullvalene.
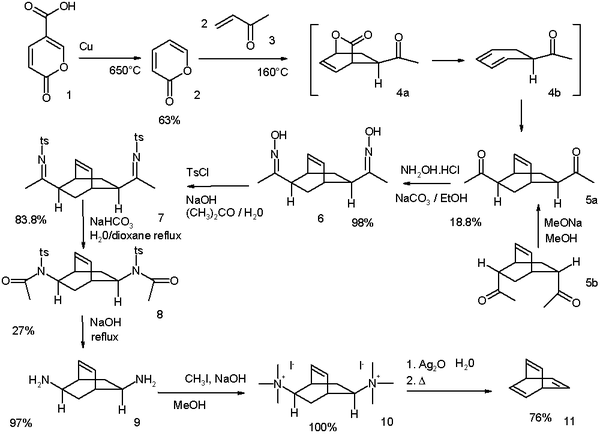
The original Zimmerman synthesis modified in 1969 starts from coumalic acid:
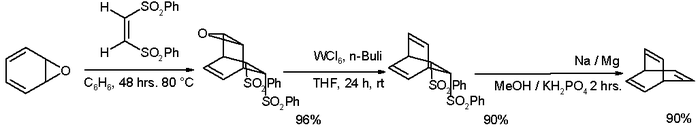
many alternative routes have been devised since then, one of them starting from oxepin
:
An alternate route that allows synthesis of the parent barrelene system and a variety of substituted barrelenes has also been reported
with hydrogen gas and Adams' catalyst
in ethanol
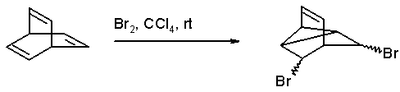
to the fully saturated bicyclo[2.2.2]-octane. Bromination with bromine
in tetrachloromethane gives a di-bromo adduct because a coupling reaction
intervenes:
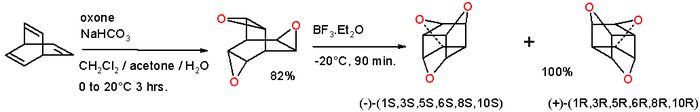
Epoxidation of barrelene with oxone gives the trioxatrishomobarrelene which on rearrangement
with boron trifluoride
(driving force:relief of strain energy
) converts into the trioxatrishomocubane:
This compound can be envisioned as a cubane
with three oxygen atoms inserted into three opposite edges or as 9-crown-3 capped by two methine
units. The molecule is chiral
and the separate enantiomer
s have been isolated.
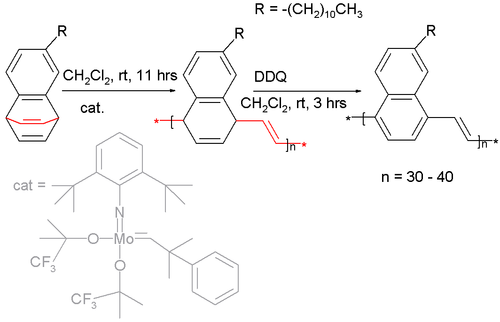
Certain barrelenes have been used as a monomer
in a ring opening metathesis polymerization :
The catalyst is a Fischer carbene (a molybdenum bis-(hexafluoro-tert-butoxy) carbene catalyst) and the long alkyl chain attached to the monomer is required for solubility. Oxidation of the polymer with DDQ
affords the naphthalene pendant of poly(p-phenylene vinylene)
.
Isopentane
solutions of barrelene undergo photolytic isomerisation
when acetone
is added as a photosensitizer to produce semibullvalene. Prolonged irradiation results in further isomerisation to form cyclooctatetraene
.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
C8H8 and systematic name
Systematic name
A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection...
bicyclo[2.2.2]octa-2,5,7-triene. First synthesized and described by H. E. Zimmerman
Howard Zimmerman
Howard E. Zimmerman is a professor of chemistry at the University of Wisconsin–Madison. He was elected to the National Academy of Sciences in 1980 and the recipient of the 1985 American Institute of Chemists Pioneering Award....
in 1960 the name derives from the obvious resemblance with a barrel
Barrel
A barrel or cask is a hollow cylindrical container, traditionally made of vertical wooden staves and bound by wooden or metal hoops. Traditionally, the barrel was a standard size of measure referring to a set capacity or weight of a given commodity. A small barrel is called a keg.For example, a...
, with the staves being three ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
units attached to two methine
Methine
In chemistry, methine is a trivalent functional group CH, derived formally from methane. The methine group consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen...
groups. It is the formal Diels-Alder adduct of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
. Due to its unusual molecular geometry
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
the compound is of considerable interest to theoretical chemists. Like benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, barrelene has a set of 6 cyclic, but not planar, overlapping p-orbitals. Because it is not possible to avoid a destabilizing overlap of opposite-sign lobes, the structure represents Möbius aromaticity
Möbius aromaticity
In organic chemistry, Möbius aromaticity is a special type of aromaticity believed to exist in a number of organic molecules. In terms of MO theory these compounds have in common a monocyclic array of molecular orbitals in which there is an odd number of out-of-phase overlaps which reveals the...
.
Triptycene
Triptycene
Triptycenes are a class of aromatic hydrocarbons. The parent compound triptycene is the Diels-Alder reaction product of anthracene and benzyne. The compound has a paddlewheel configuration with D3h symmetry...
s, with the alkene groups part of an arene, are related compounds. It is also a starting material for many other organic compounds such as semibullvalene.
The original Zimmerman synthesis modified in 1969 starts from coumalic acid:
many alternative routes have been devised since then, one of them starting from oxepin
Oxepin
Oxepin is an oxygen-containing heterocycle consisting of a seven-membered ring with three double bonds. It exists as an equilibrium mixture with benzene oxide. The oxepin-benzene oxide system has fluctuating bonds in which the equilibrium can be shifted to one extreme or the other by suitable...
:
An alternate route that allows synthesis of the parent barrelene system and a variety of substituted barrelenes has also been reported
Barrelene Reactions
Barrelene is hydrogenatedHydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
with hydrogen gas and Adams' catalyst
Adams' catalyst
Adams' catalyst, also known as platinum dioxide, is usually represented as platinum oxide hydrate, PtO2-H2O. It is a catalyst for hydrogenation and hydrogenolysis in organic synthesis. This dark brown powder is commercially available...
in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
to the fully saturated bicyclo[2.2.2]-octane. Bromination with bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
in tetrachloromethane gives a di-bromo adduct because a coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
intervenes:
Epoxidation of barrelene with oxone gives the trioxatrishomobarrelene which on rearrangement
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
with boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
(driving force:relief of strain energy
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
) converts into the trioxatrishomocubane:
This compound can be envisioned as a cubane
Cubane
Cubane is a synthetic hydrocarbon molecule that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons. It was first synthesized in 1964 by Philip Eaton, a...
with three oxygen atoms inserted into three opposite edges or as 9-crown-3 capped by two methine
Methine
In chemistry, methine is a trivalent functional group CH, derived formally from methane. The methine group consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen...
units. The molecule is chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
and the separate enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s have been isolated.
Certain barrelenes have been used as a monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
in a ring opening metathesis polymerization :
The catalyst is a Fischer carbene (a molybdenum bis-(hexafluoro-tert-butoxy) carbene catalyst) and the long alkyl chain attached to the monomer is required for solubility. Oxidation of the polymer with DDQ
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone is the chemical reagent with formula C8Cl2N2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols and steroid ketones in organic chemistry. DDQ decomposes in water, but is stable in aqueous mineral acid.-Preparation:Synthesis of DDQ...
affords the naphthalene pendant of poly(p-phenylene vinylene)
Poly(p-phenylene vinylene)
Poly is a conducting polymer of the rigid-rod polymer host family.PPV is the only polymer of this type that has so far been successfully processed into a highly ordered crystalline thin film. PPV and its derivatives are conducting polymers of rigid-rod polymer family...
.
Isopentane
Isopentane
Isopentane, C5H12, also called methylbutane or 2-methylbutane, is a branched-chain alkane with five carbon atoms. Isopentane is an extremely volatile and extremely flammable liquid at room temperature and pressure. The normal boiling point is just a few degrees above room temperature and...
solutions of barrelene undergo photolytic isomerisation
Isomerisation
In chemistry isomerisation is the process by which one molecule is transformed into another molecule which has exactly the same atoms, but the atoms are rearranged e.g. A-B-C → B-A-C . In some molecules and under some conditions, isomerisation occurs spontaneously...
when acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
is added as a photosensitizer to produce semibullvalene. Prolonged irradiation results in further isomerisation to form cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
.