Cubane
Encyclopedia
Cubane is a synthetic hydrocarbon
molecule
that consists of eight carbon
atom
s arranged at the corners of a cube, with one hydrogen
atom attached to each carbon atom. A solid crystal
line substance, cubane is one of the Platonic hydrocarbons. It was first synthesized in 1964 by Philip Eaton
, a professor of chemistry at the University of Chicago
. Before Eaton and Cole's work, researchers believed that cubic carbon-based molecules could not exist, because the unusually sharp 90-degree bonding angle of the carbon atoms were expected to be too highly strained
, and hence unstable. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths.
The other Platonic hydrocarbons are dodecahedrane
and tetrahedrane
.
Cubane and its derivative compounds have many important properties. The 90-degree bonding angle of the carbon atoms in cubane means that the bonds are highly strained. Therefore, cubane compounds are highly reactive, which in principle may make them useful as high-density, high-energy fuel
s and explosives (for example, octanitrocubane
and heptanitrocubane
).
Cubane also has the highest density of any hydrocarbon, further contributing to its ability to store large amounts of energy, which would reduce the size and weight of fuel tanks in aircraft and especially rocket boosters. Researchers are looking into using cubane and similar cubic molecules in medicine
and nanotechnology
.
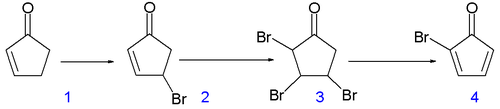
is a classic and starts from 2-cyclopentenone (compound 1.1 in scheme 1):
Reaction with N-bromosuccinimide
in carbon tetrachloride
places an allylic bromine atom in 1.2 and further bromination with bromine
in pentane
- methylene chloride gives the tribromide 1.3. Two equivalents of hydrogen bromide
are eliminated
from this compound with diethylamine
in diethyl ether
to bromocyclopentadienone 1.4
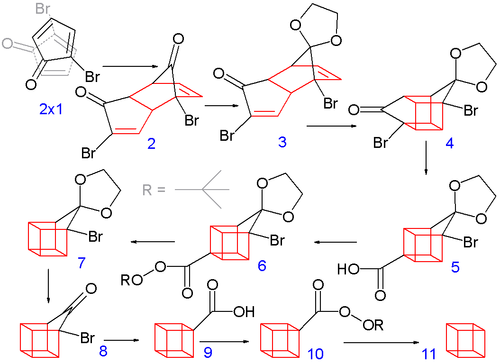
In the second part (scheme 2), the spontaneous Diels-Alder dimerization
of 2.1 to 2.2 is analogous to the dimerization of cyclopentadiene
to dicyclopentadiene
. For the next steps to succeed, only the endo isomer should form; this happens because the bromine atoms, on their approach, take up positions as far away from each other, and from the carbonyl group, as possible. In this way the like-dipole interactions are minimized in the transition state
for this reaction step. Both carbonyl
groups are protected
as acetal
s with ethylene glycol
and p-toluenesulfonic acid
in benzene
; one acetal is then selectively deprotected with aqueous hydrochloric acid
to 2.3
In the next step, the endo isomer 2.3 (with both alkene
groups in close proximity) forms the cage-like isomer 2.4 in a photochemical [2+2] cycloaddition
. The bromoketone
group is converted to ring-contracted carboxylic acid
2.5 in a Favorskii rearrangement
with potassium hydroxide
. Next, the thermal decarboxylation
takes place through the acid chloride (with thionyl chloride
) and the tert-butyl perester 2.6 (with t-butyl hydroperoxide and pyridine
) to 2.7; afterward, the acetal is once more removed in 2.8. A second Favorskii rearrangement gives 2.9, and finally another decarboxylation gives 2.10 and 2.11.
s. Such species contain sulfur and Fe at alternating corners. Alternatively such inorganic cube clusters can often be viewed as interpenetrated S4 and Fe4 tetrahedra. Many organometallic compounds adopt cube structures, examples being (Cp
Fe)4(CO)4, (Cp*Ru)4Cl4, and (Ph3P
Ag)4I4,
may be produced from cubane by a metal-ion-catalyzed σ-bond rearrangement.
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
that consists of eight carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s arranged at the corners of a cube, with one hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom attached to each carbon atom. A solid crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
line substance, cubane is one of the Platonic hydrocarbons. It was first synthesized in 1964 by Philip Eaton
Philip Eaton
Philip E. Eaton is a Professor Emeritus of Chemistry at the University of Chicago. He and his fellow researchers were the first to synthesize the "impossible" cubane molecule in 1964....
, a professor of chemistry at the University of Chicago
University of Chicago
The University of Chicago is a private research university in Chicago, Illinois, USA. It was founded by the American Baptist Education Society with a donation from oil magnate and philanthropist John D. Rockefeller and incorporated in 1890...
. Before Eaton and Cole's work, researchers believed that cubic carbon-based molecules could not exist, because the unusually sharp 90-degree bonding angle of the carbon atoms were expected to be too highly strained
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional...
, and hence unstable. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths.
The other Platonic hydrocarbons are dodecahedrane
Dodecahedrane
Dodecahedrane is a chemical compound first synthesised by Leo Paquette of Ohio State University in 1982, primarily for the "aesthetically pleasing symmetry of the dodecahedral framework"....
and tetrahedrane
Tetrahedrane
Tetrahedrane is a platonic hydrocarbon with chemical formula 44 and a tetrahedral structure. Extreme angle strain prevents this molecule from forming naturally....
.
Cubane and its derivative compounds have many important properties. The 90-degree bonding angle of the carbon atoms in cubane means that the bonds are highly strained. Therefore, cubane compounds are highly reactive, which in principle may make them useful as high-density, high-energy fuel
Fuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
s and explosives (for example, octanitrocubane
Octanitrocubane
Octanitrocubane is a high explosive that, like TNT, is shock-insensitive , not detonating even when struck by a hammer...
and heptanitrocubane
Heptanitrocubane
Heptanitrocubane is a new experimental high explosive based on the cubic eight-carbon cubane molecule and closely related to octanitrocubane. Seven of the eight hydrogen atoms at the corners of the cubane molecule are replaced by nitro groups, giving the final molecular formula .As with...
).
Cubane also has the highest density of any hydrocarbon, further contributing to its ability to store large amounts of energy, which would reduce the size and weight of fuel tanks in aircraft and especially rocket boosters. Researchers are looking into using cubane and similar cubic molecules in medicine
Medicine
Medicine is the science and art of healing. It encompasses a variety of health care practices evolved to maintain and restore health by the prevention and treatment of illness....
and nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
.
Synthesis
The original 1964 cubane organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
is a classic and starts from 2-cyclopentenone (compound 1.1 in scheme 1):
Reaction with N-bromosuccinimide
N-Bromosuccinimide
N-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution and electrophilic addition reactions in organic chemistry. NBS can be considered a convenient source of cationic bromine.-Preparation:...
in carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
places an allylic bromine atom in 1.2 and further bromination with bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
in pentane
Pentane
Pentane is an organic compound with the formula C5H12 — that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two being called...
- methylene chloride gives the tribromide 1.3. Two equivalents of hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
are eliminated
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
from this compound with diethylamine
Diethylamine
Diethylamine is a secondary amine with the molecular structure CH3CH2NHCH2CH3. It is a flammable, strongly alkaline liquid. It is miscible with water and ethanol. It is a colorless liquid which often appears brown due to impurities...
in diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
to bromocyclopentadienone 1.4
In the second part (scheme 2), the spontaneous Diels-Alder dimerization
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
of 2.1 to 2.2 is analogous to the dimerization of cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
to dicyclopentadiene
Dicyclopentadiene
Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white crystalline solid with a camphor-like odor. Its energy density is 10,975 Wh/l....
. For the next steps to succeed, only the endo isomer should form; this happens because the bromine atoms, on their approach, take up positions as far away from each other, and from the carbonyl group, as possible. In this way the like-dipole interactions are minimized in the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
for this reaction step. Both carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups are protected
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
as acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
s with ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
and p-toluenesulfonic acid
P-Toluenesulfonic acid
p-Toluenesulfonic acid or tosylic acid is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The 4-CH3C6H4SO2- group is known as the Tosyl group and is often abbreviated as Ts or Tos...
in benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
; one acetal is then selectively deprotected with aqueous hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
to 2.3
In the next step, the endo isomer 2.3 (with both alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
groups in close proximity) forms the cage-like isomer 2.4 in a photochemical [2+2] cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
. The bromoketone
Haloketone
A haloketone in organic chemistry is a functional group consisting of a ketone group or more general a carbonyl group with a α-halogen substituent. The general structure is RR'CCR where R is an alkyl or aryl residue and X any one of the halogens...
group is converted to ring-contracted carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
2.5 in a Favorskii rearrangement
Favorskii rearrangement
The Favorskii rearrangement , named for the Russian chemist Alexei Yevgrafovich Favorskii, is most principally a rearrangement of cyclopropanones and α-halo ketones which leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorski rearrangement constitutes a ring...
with potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
. Next, the thermal decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
takes place through the acid chloride (with thionyl chloride
Thionyl chloride
Thionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
) and the tert-butyl perester 2.6 (with t-butyl hydroperoxide and pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
) to 2.7; afterward, the acetal is once more removed in 2.8. A second Favorskii rearrangement gives 2.9, and finally another decarboxylation gives 2.10 and 2.11.
Inorganic cubes and related derivatives
The cube motif occurs outside of the area of organic chemistry. Prevalent non-organic cubes are the [Fe4-S4] clusters found pervasively iron-sulfur proteinIron-sulfur protein
Iron-sulfur proteins are proteins characterized by the presence of iron-sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states...
s. Such species contain sulfur and Fe at alternating corners. Alternatively such inorganic cube clusters can often be viewed as interpenetrated S4 and Fe4 tetrahedra. Many organometallic compounds adopt cube structures, examples being (Cp
Cyclopentadienyl
In organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
Fe)4(CO)4, (Cp*Ru)4Cl4, and (Ph3P
Triphenylphosphine
Triphenylphosphine is a common organophosphorus compound with the formula P3 - often abbreviated to PPh3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature...
Ag)4I4,
Reactions
CuneaneCuneane
Cuneane is a saturated hydrocarbon. Its name is derived from the Latin “cuneus”, meaning a wedge. Cuneane may be produced from cubane by metal-ion-catalyzed σ-bond rearrangement. Similar reactions are known for homocubane and bishomocubane...
may be produced from cubane by a metal-ion-catalyzed σ-bond rearrangement.