
Beta-lactoglobulin
Encyclopedia
Whey protein
Whey protein is a mixture of globular proteins isolated from whey, the liquid material created as a by-product of cheese production. Some preclinical studies in rodents have suggested that whey protein may possess anti-inflammatory or anti-cancer properties; however, human data is lacking...
of cow and sheep's milk
Milk
Milk is a white liquid produced by the mammary glands of mammals. It is the primary source of nutrition for young mammals before they are able to digest other types of food. Early-lactation milk contains colostrum, which carries the mother's antibodies to the baby and can reduce the risk of many...
(~3 g/l), and is also present in many other mammalian species; a notable exception being humans. Its structure, properties and biological role have been reviewed many times .
Structure and role
Unlike the other main whey protein, α-lactalbuminAlpha-lactalbumin
Lactalbumin, alpha-, also known as LALBA, is a protein that in humans is encoded by the LALBA gene.- Function :α-Lactalbumin is an important whey protein in cow's milk , and is also present in the milk of many other mammalian species...
, no clear function has been identified for β-lactoglobulin, although it binds to several hydrophobic molecules, suggesting its role in their transport. The strong suggestion is that the molecule exists primarily as a food source. Several genetic variants have been identified, the main ones in the cow being labelled A and B. Because of its abundance and ease of purification, it has been subjected to a wide range of biophysical studies. Its structure has been determined several times by X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
and NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
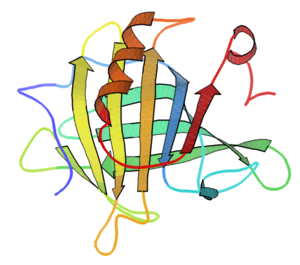
. One such structure is shown on the right (from http://www.pdb.org entry 3BLG). β-lactoglobulin is of direct interest to the food industry
Food industry
The food production is a complex, global collective of diverse businesses that together supply much of the food energy consumed by the world population...
since its properties can variously be advantageous or disadvantageous in dairy
Dairy
A dairy is a business enterprise established for the harvesting of animal milk—mostly from cows or goats, but also from buffalo, sheep, horses or camels —for human consumption. A dairy is typically located on a dedicated dairy farm or section of a multi-purpose farm that is concerned...
products and processing .
Bovine β-lactoglobulin is a relatively small protein of 162 residue
Residue (chemistry)
In chemistry, residue is the material remaining after a distillation or an evaporation, or to a portion of a larger molecule, such as a methyl group. It may also refer to the undesired byproducts of a reaction....
s, with an 18.4 kDa molecular weight (1 dalton being defined as 1 unified atomic mass unit). In physiological condition
Physiological condition
Physiological condition or, more often "physiological conditions" is a term used in biology, biochemistry, and medicine. It refers to conditions of the external or internal milieu that may occur in nature for that organism or cell system, in contrast to artificial laboratory conditions...
s it is predominantly dimeric, but dissociates to a monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
below about pH 3. Nevertheless, its native state
Native state
In biochemistry, the native state of a protein is its operative or functional form. While all protein molecules begin as simple unbranched chains of amino acids, once completed they assume highly specific three-dimensional shapes; that ultimate shape, known as tertiary structure, is the folded...
remains fairly intact at lower pH values, as determined using NMR .
β-Lactoglobulin solutions form gel
Gel
A gel is a solid, jelly-like material that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state...
s in various conditions, when the native structure is sufficiently destabilised to allow aggregation . Under prolonged heating at low pH and low ionic strength
Ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
, a transparent `fine-stranded' gel is formed, in which the protein molecules assemble into long stiff fibres.
Folding intermediates for this protein can be studied using light spectroscopy and denaturant. Such experiments interestingly show an unusual but important intermediate composed purely of alpha helices, despite the fact that the native structure is beta sheet. Evolution has probably selected for the helical intermediate to avoid aggregation during the folding process.
As milk is a known allergen (as listed in Annex IIIa of Directive 2000/13/EC), manufacturers need to prove the presence or absence of β-lactoglobulin to ensure their labelling satisfies the requirements of the aforementioned directive. Food testing laboratories can use ELISA (enzyme linked immunosorbent assay) methods to identify and quantify β-lactoglobulin in food products.
Footnotes
- Hambling, S. G., A. S. McAlpine, and L. Sawyer. 1992. Advanced Dairy Chemistry: 1. Proteins, chapter: Beta-lactoglobulin. Elsevier Applied Science, 141–190.
- Sawyer, L., and G. Kontopidis. 2000. The core lipocalin, bovine beta-lactoglobulin. Biochim Biophys Acta 1482:136–48.
- Kontopidis, G., C. Holt, and L. Sawyer. 2004. Invited review: beta-lactoglobulin: binding properties, structure, and function. J Dairy Sci 87:785–96.
- Jost, R. 1993. Functional characteristics of dairy proteins. Trends in Food Science & Technology 4:283–288.
- Uhrinova, S., M. H. Smith, G. B. Jameson, D. Uhrin, L. Sawyer, and P. N. Barlow. 2000. Structural changes accompanying ph-induced dissociation of the beta-lactoglobulin dimer. Biochemistry 39:3565–74.
- Bromley, E. H. C., M. R. H. Krebs, and A. M. Donald. 2005. Aggregation across the length scales in beta-lactoglobulin. Faraday Discussions. 128:13–27.
- Kuwajima K., Yamaya H. & Sugai S. 1996. The Burst-phase Intermediate in the Refolding of beta-Lactoglobulin Studied by Stopped-flow Circular Dichroism and Absorption Spectroscopy. Journal of Molecular Biology, 264:806-822.

