
Bile acid
Encyclopedia
Bile acids are steroid
acid
s found predominantly in the bile
of mammals. Bile salts are bile acids compounded with a cation, usually sodium
. In humans, the salts of taurocholic acid
and glycocholic acid
(derivatives of cholic acid
) represent approximately eighty percent of all bile salts. The two major bile acids are cholic acid
, and chenodeoxycholic acid
. Bile acids, glycine
and taurine conjugates, and 7-alpha-dehydroxylated derivatives (deoxycholic acid
and lithocholic acid
) are all found in human intestinal bile. An increase in bile flow is exhibited with an increased secretion of bile acids. The main function of bile acid is to facilitate the formation of micelles, which promotes processing of dietary fat.
by the cytochrome P450-mediated oxidation of cholesterol
. They are conjugated with taurine or the amino acid glycine
, or with a sulfate
or a glucuronide
, and are then stored in the gallbladder
, which concentrates the salts by removing the water. In humans, the rate limiting step is the addition of a hydroxyl group on position 7 of the steroid nucleus by the enzyme cholesterol 7 alpha-hydroxylase. Upon eating a meal, the contents of the gallbladder are secreted into the intestine
, where bile acids serve the purpose of emulsifying dietary fats. Bile acids serve other functions, including eliminating cholesterol from the body, driving the flow of bile to eliminate catabolites from the liver, emulsifying lipids and fat soluble vitamins in the intestine to form micelles that can be transported via the lacteal system, and aiding in the reduction of the bacteria flora found in the small intestine and biliary tract.
Bile acid refers to the protonated (-COOH) form. Bile salt refers to the deprotonated or ionized (-COO-) form. Conjugated bile acids are more efficient at emulsifying fats because at intestinal pH, they are more ionized than unconjugated bile acids.
Synthesis of bile acids is a major route of cholesterol metabolism in most species other than humans. The body produces about 800 mg of cholesterol per day and about half of that is used for bile acid synthesis. In total about 20-30 grams of bile acids are secreted into the intestine
daily. About 90% of excreted bile acids are reabsorbed by active transport
in the ileum
and recycled in what is referred to as the enterohepatic circulation
which moves the bile salts from the intestinal system back to the liver and the gallbladder. This allows a low rate of daily synthesis, but high secretion to the digestive system. Bile is also used to break down fat globules into tiny droplets. Bile from slaughtered animals can be used in the preparation of soap.
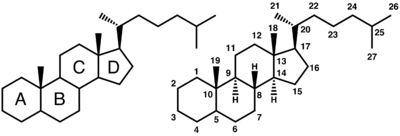 Bile salts constitute a large family of molecules, composed of a steroid structure with four rings, a five or eight carbon side-chain terminating in a carboxylic acid, and the presence and orientation of different numbers of hydroxyl groups. The four rings are labeled from left to right (as commonly drawn) A, B, C, and D, with the D-ring being smaller by one carbon than the other three. The hydroxyl groups have a choice of being in 2 positions, either up (or out) termed beta (often drawn by convention as a solid line), or down, termed alpha (seen as a dashed line in drawings). All bile acids have a hydroxyl group on position 3, which was derived from the parent molecule, cholesterol. In cholesterol, the 4 steroid rings are flat and the position of the 3-hydroxyl is beta.
Bile salts constitute a large family of molecules, composed of a steroid structure with four rings, a five or eight carbon side-chain terminating in a carboxylic acid, and the presence and orientation of different numbers of hydroxyl groups. The four rings are labeled from left to right (as commonly drawn) A, B, C, and D, with the D-ring being smaller by one carbon than the other three. The hydroxyl groups have a choice of being in 2 positions, either up (or out) termed beta (often drawn by convention as a solid line), or down, termed alpha (seen as a dashed line in drawings). All bile acids have a hydroxyl group on position 3, which was derived from the parent molecule, cholesterol. In cholesterol, the 4 steroid rings are flat and the position of the 3-hydroxyl is beta.
In many species, the initial step in the formation of a bile acid is the addition of a 7-alpha hydroxyl group. Subsequently, in the conversion from cholesterol to a bile acid, the junction between the first two steroid rings (A and B) is altered, making the molecule bent, and in this process, the 3-hydroxyl is converted to the alpha orientation. Thus, the default simplest bile acid (of 24 carbons) has two hydroxyl groups at positions 3-alpha and 7-alpha. The chemical name for this compound is 3-alpha,7-alpha-dihydroxy-5-beta-cholan-24-oic acid, or as it is commonly known, chenodeoxycholic acid. This bile acid was first isolated from the domestic goose, from which the "cheno" portion of the name was derived.
Another bile acid, cholic acid (with 3 hydroxyl groups) had already been described, so the discovery of chenodeoxcholic acid (with 2 hydroxyl groups) made the new bile acid a "deoxycholic acid" in that it had one less hydroxyl group than cholic acid. The 5-beta portion of the name denotes the orientation of the junction between rings A and B of the steroid nucleus (in this case, they are bent). The term "cholan" denotes a particular steroid structure of 24 carbons, and the "24-oic acid" indicates that the carboxylic acid is found at position 24, which happens to be at the end of the side-chain. Chenodeoxycholic acid is made by many species, and is quite a functional bile acid. Its chief drawback lies in the ability of intestinal bacteria to remove the 7-alpha hydroxyl group, a process termed dehydroxylation. The resulting bile acid has only a 3-alpha hydroxyl group and is termed lithocholic acid (litho = stone). It is poorly water-soluble and rather toxic to cells. Bile acids formed by synthesis in the liver are termed "primary" bile acids, and those made by bacteria are termed "secondary" bile acids. As a result, chenodeoxycholic acid is a primary bile acid, and lithocholic acid is a secondary bile acid.
To avoid the problems associated with the production of lithocholic acid, most species add a third hydroxyl group to chenodeoxycholic acid. In this manner, the subsequent removal of the 7-alpha hydroxyl group by intestinal bacteria will result in a less toxic, still functional dihydroxy bile acid. Over the course of vertebrate evolution, a number of positions have been chosen for placement of the third hydroxyl group. Initially, the 16-alpha position was favored, particularly in birds. Later, this position was superseded by a large number of species selecting position 12-alpha. Primates (including humans) utilize 12-alpha for their third hydroxyl group position. The resulting primary bile acid in humans is 3-alpha,7-alpha,12-alpha-trihydroxy-5-beta-cholan-24-oic acid, or as it is commonly called, cholic acid.
In the intestine, cholic acid is dehydroxylated to form the dihydroxy bile acid deoxycholic acid. In many vertebrate orders still subject to speciation, new species are discarding 12-alpha hydroxylation in favor of a hydroxy group on position 23 of the side-chain. Vertebrate families and species exist that have experimented with and utilize just about every position imaginable on the steroid nucleus and side-chain.
The principal bile acids are:
In humans, the two primary bile acids are cholic acid
and chenodeoxycholic acid
. Deoxycholic acid
and lithocholic acid
are the two secondary bile acids found in lower concentrations, resulting from bacterial action in the intestine which are absorbed and resecreted by the liver.
Prior to secretion by the liver, bile acids are conjugated with either the amino acid glycine or taurine. Conjugation increases water solubility, preventing passive re-absorption once secreted into the small intestine. As a result, the concentration of bile acids in the small intestine can stay high enough to form micelles and solubilize lipids. "Critical micellar concentration" refers to both an intrinsic property of the bile acid itself and amount of bile acid necessary to function in the spontaneous and dynamic formation of micelles.
Bile acids can also be thought of as steroid hormones, secreted from the liver and having direct metabolic actions in the body through the nuclear receptor FXR
, or the cell membrane receptor TGR5 .
s or detergent
s, bile acids are potentially toxic to cells, and their concentrations are tightly regulated. They function as a signaling molecule
in the liver
and the intestines by activating a nuclear hormone
receptor, FXR
, also known by its gene name . Activation of FXR in the liver inhibits synthesis of bile acids, and is one mechanism of feedback control when bile acid levels are too high. FXR activation by bile acids during absorption in the intestine increasees transcription and synthesis of FGF19
, which will then inhibit bile acid synthesis in the liver. Emerging evidence associates FXR activation with alterations in triglyceride
metabolism
, glucose metabolism, and liver
growth.
s bind bile acids in the gut, preventing reabsorption. In so doing, more endogenous cholesterol is shunted into the production of bile acids, thereby lowering cholesterol levels. The sequestered bile acids are then excreted in the feces.
Tests for bile acids are useful in both human and veterinary medicine, as they help to diagnose a number of conditions, including cholestasis
, portosystemic shunt
, and hepatic microvascular dysplasia
.
Excess concentrations of bile acids in the colon are a cause of chronic diarrhea. This condition of bile acid malabsorption
can be diagnosed by the SeHCAT
test and treated with bile acid sequestrants .
The role of black bile in carcinogenesis was suggested already by Hippocrates. Today it's well-documented that bile acids are carcinogens and tumor promotors in experimental models. Their role in carcinogenesis is best documented in Barrett's esophagus
and adenocarcinoma at the gastroesophageal junctions.
Steroid
A steroid is a type of organic compound that contains a characteristic arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary fat cholesterol, the sex hormones estradiol and testosterone, and the anti-inflammatory drug dexamethasone.The core...
acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s found predominantly in the bile
Bile
Bile or gall is a bitter-tasting, dark green to yellowish brown fluid, produced by the liver of most vertebrates, that aids the process of digestion of lipids in the small intestine. In many species, bile is stored in the gallbladder and upon eating is discharged into the duodenum...
of mammals. Bile salts are bile acids compounded with a cation, usually sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
. In humans, the salts of taurocholic acid
Taurocholic acid
Taurocholic acid, known also as cholaic acid, cholyltaurine, or acidum cholatauricum, is a deliquescent yellowish crystalline bile acid involved in the emulsification of fats. It occurs as a sodium salt in the bile of mammals. It is a conjugate of cholic acid with taurine...
and glycocholic acid
Glycocholic acid
Glycocholic acid, or cholylglycine, is a crystalline bile acid involved in the emulsification of fats. It occurs as a sodium salt in the bile of mammals. It is a conjugate of cholic acid with glycine. Its anion is called glycocholate....
(derivatives of cholic acid
Cholic acid
Cholic acid is a bile acid, a white crystalline substance insoluble in water , with a melting point of 200-201 °C. Salts of cholic acid are called cholates. Cholic acid, along with chenodeoxycholic acid, is one of two major bile acids produced by the liver where it is synthesized from cholesterol...
) represent approximately eighty percent of all bile salts. The two major bile acids are cholic acid
Cholic acid
Cholic acid is a bile acid, a white crystalline substance insoluble in water , with a melting point of 200-201 °C. Salts of cholic acid are called cholates. Cholic acid, along with chenodeoxycholic acid, is one of two major bile acids produced by the liver where it is synthesized from cholesterol...
, and chenodeoxycholic acid
Chenodeoxycholic acid
Chenodeoxycholic acid is a bile acid. It occurs as a white crystalline substance insoluble in water but soluble in alcohol and acetic acid, with melting point at 165-167 °C. Salts of this carboxylic acid are called chenodeoxycholates...
. Bile acids, glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
and taurine conjugates, and 7-alpha-dehydroxylated derivatives (deoxycholic acid
Deoxycholic acid
Deoxycholic acid, also known as deoxycholate, cholanoic acid, and 3α,12α-dihydroxy-5β-cholanate, is a bile acid. Deoxycholic acid is one of the secondary bile acids, which are metabolic byproducts of intestinal bacteria. The two primary bile acids secreted by the liver are cholic acid and...
and lithocholic acid
Lithocholic acid
Lithocholic acid is a bile acid that acts as a detergent to solubilize fats for absorption. It is made from chenodeoxycholic acid by bacterial action in the colon.It has been implicated in human and experimental animal carcinogenesis....
) are all found in human intestinal bile. An increase in bile flow is exhibited with an increased secretion of bile acids. The main function of bile acid is to facilitate the formation of micelles, which promotes processing of dietary fat.
Production and distribution
Bile acids are made in the liverLiver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
by the cytochrome P450-mediated oxidation of cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
. They are conjugated with taurine or the amino acid glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
, or with a sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
or a glucuronide
Glucuronide
A glucuronide, also known as glucuronoside, is any substance produced by linking glucuronic acid to another substance via a glycosidic bond...
, and are then stored in the gallbladder
Gallbladder
In vertebrates the gallbladder is a small organ that aids mainly in fat digestion and concentrates bile produced by the liver. In humans the loss of the gallbladder is usually easily tolerated....
, which concentrates the salts by removing the water. In humans, the rate limiting step is the addition of a hydroxyl group on position 7 of the steroid nucleus by the enzyme cholesterol 7 alpha-hydroxylase. Upon eating a meal, the contents of the gallbladder are secreted into the intestine
Intestine
In human anatomy, the intestine is the segment of the alimentary canal extending from the pyloric sphincter of the stomach to the anus and, in humans and other mammals, consists of two segments, the small intestine and the large intestine...
, where bile acids serve the purpose of emulsifying dietary fats. Bile acids serve other functions, including eliminating cholesterol from the body, driving the flow of bile to eliminate catabolites from the liver, emulsifying lipids and fat soluble vitamins in the intestine to form micelles that can be transported via the lacteal system, and aiding in the reduction of the bacteria flora found in the small intestine and biliary tract.
Bile acid refers to the protonated (-COOH) form. Bile salt refers to the deprotonated or ionized (-COO-) form. Conjugated bile acids are more efficient at emulsifying fats because at intestinal pH, they are more ionized than unconjugated bile acids.
Synthesis of bile acids is a major route of cholesterol metabolism in most species other than humans. The body produces about 800 mg of cholesterol per day and about half of that is used for bile acid synthesis. In total about 20-30 grams of bile acids are secreted into the intestine
Intestine
In human anatomy, the intestine is the segment of the alimentary canal extending from the pyloric sphincter of the stomach to the anus and, in humans and other mammals, consists of two segments, the small intestine and the large intestine...
daily. About 90% of excreted bile acids are reabsorbed by active transport
Active transport
Active transport is the movement of a substance against its concentration gradient . In all cells, this is usually concerned with accumulating high concentrations of molecules that the cell needs, such as ions, glucose, and amino acids. If the process uses chemical energy, such as from adenosine...
in the ileum
Ileum
The ileum is the final section of the small intestine in most higher vertebrates, including mammals, reptiles, and birds. In fish, the divisions of the small intestine are not as clear and the terms posterior intestine or distal intestine may be used instead of ileum.The ileum follows the duodenum...
and recycled in what is referred to as the enterohepatic circulation
Enterohepatic circulation
Enterohepatic circulation refers to the circulation of biliary acids from the liver, where they are produced and secreted in the bile, to the small intestine, where it aids in digestion of fats and other substances, back to the liver....
which moves the bile salts from the intestinal system back to the liver and the gallbladder. This allows a low rate of daily synthesis, but high secretion to the digestive system. Bile is also used to break down fat globules into tiny droplets. Bile from slaughtered animals can be used in the preparation of soap.
Types

In many species, the initial step in the formation of a bile acid is the addition of a 7-alpha hydroxyl group. Subsequently, in the conversion from cholesterol to a bile acid, the junction between the first two steroid rings (A and B) is altered, making the molecule bent, and in this process, the 3-hydroxyl is converted to the alpha orientation. Thus, the default simplest bile acid (of 24 carbons) has two hydroxyl groups at positions 3-alpha and 7-alpha. The chemical name for this compound is 3-alpha,7-alpha-dihydroxy-5-beta-cholan-24-oic acid, or as it is commonly known, chenodeoxycholic acid. This bile acid was first isolated from the domestic goose, from which the "cheno" portion of the name was derived.
Another bile acid, cholic acid (with 3 hydroxyl groups) had already been described, so the discovery of chenodeoxcholic acid (with 2 hydroxyl groups) made the new bile acid a "deoxycholic acid" in that it had one less hydroxyl group than cholic acid. The 5-beta portion of the name denotes the orientation of the junction between rings A and B of the steroid nucleus (in this case, they are bent). The term "cholan" denotes a particular steroid structure of 24 carbons, and the "24-oic acid" indicates that the carboxylic acid is found at position 24, which happens to be at the end of the side-chain. Chenodeoxycholic acid is made by many species, and is quite a functional bile acid. Its chief drawback lies in the ability of intestinal bacteria to remove the 7-alpha hydroxyl group, a process termed dehydroxylation. The resulting bile acid has only a 3-alpha hydroxyl group and is termed lithocholic acid (litho = stone). It is poorly water-soluble and rather toxic to cells. Bile acids formed by synthesis in the liver are termed "primary" bile acids, and those made by bacteria are termed "secondary" bile acids. As a result, chenodeoxycholic acid is a primary bile acid, and lithocholic acid is a secondary bile acid.
To avoid the problems associated with the production of lithocholic acid, most species add a third hydroxyl group to chenodeoxycholic acid. In this manner, the subsequent removal of the 7-alpha hydroxyl group by intestinal bacteria will result in a less toxic, still functional dihydroxy bile acid. Over the course of vertebrate evolution, a number of positions have been chosen for placement of the third hydroxyl group. Initially, the 16-alpha position was favored, particularly in birds. Later, this position was superseded by a large number of species selecting position 12-alpha. Primates (including humans) utilize 12-alpha for their third hydroxyl group position. The resulting primary bile acid in humans is 3-alpha,7-alpha,12-alpha-trihydroxy-5-beta-cholan-24-oic acid, or as it is commonly called, cholic acid.
In the intestine, cholic acid is dehydroxylated to form the dihydroxy bile acid deoxycholic acid. In many vertebrate orders still subject to speciation, new species are discarding 12-alpha hydroxylation in favor of a hydroxy group on position 23 of the side-chain. Vertebrate families and species exist that have experimented with and utilize just about every position imaginable on the steroid nucleus and side-chain.
The principal bile acids are:
In humans, the two primary bile acids are cholic acid
Cholic acid
Cholic acid is a bile acid, a white crystalline substance insoluble in water , with a melting point of 200-201 °C. Salts of cholic acid are called cholates. Cholic acid, along with chenodeoxycholic acid, is one of two major bile acids produced by the liver where it is synthesized from cholesterol...
and chenodeoxycholic acid
Chenodeoxycholic acid
Chenodeoxycholic acid is a bile acid. It occurs as a white crystalline substance insoluble in water but soluble in alcohol and acetic acid, with melting point at 165-167 °C. Salts of this carboxylic acid are called chenodeoxycholates...
. Deoxycholic acid
Deoxycholic acid
Deoxycholic acid, also known as deoxycholate, cholanoic acid, and 3α,12α-dihydroxy-5β-cholanate, is a bile acid. Deoxycholic acid is one of the secondary bile acids, which are metabolic byproducts of intestinal bacteria. The two primary bile acids secreted by the liver are cholic acid and...
and lithocholic acid
Lithocholic acid
Lithocholic acid is a bile acid that acts as a detergent to solubilize fats for absorption. It is made from chenodeoxycholic acid by bacterial action in the colon.It has been implicated in human and experimental animal carcinogenesis....
are the two secondary bile acids found in lower concentrations, resulting from bacterial action in the intestine which are absorbed and resecreted by the liver.
Prior to secretion by the liver, bile acids are conjugated with either the amino acid glycine or taurine. Conjugation increases water solubility, preventing passive re-absorption once secreted into the small intestine. As a result, the concentration of bile acids in the small intestine can stay high enough to form micelles and solubilize lipids. "Critical micellar concentration" refers to both an intrinsic property of the bile acid itself and amount of bile acid necessary to function in the spontaneous and dynamic formation of micelles.
Bile acids can also be thought of as steroid hormones, secreted from the liver and having direct metabolic actions in the body through the nuclear receptor FXR
Farnesoid X receptor
The bile acid receptor , also known as farnesoid X receptor or NR1H4 is a nuclear receptor that is encoded by the NR1H4 gene in humans.- Function :...
, or the cell membrane receptor TGR5 .
Regulation
As surfactantSurfactant
Surfactants are compounds that lower the surface tension of a liquid, the interfacial tension between two liquids, or that between a liquid and a solid...
s or detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s, bile acids are potentially toxic to cells, and their concentrations are tightly regulated. They function as a signaling molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
in the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
and the intestines by activating a nuclear hormone
Hormone
A hormone is a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism. In essence, it is a chemical messenger that transports a signal from one...
receptor, FXR
Farnesoid X receptor
The bile acid receptor , also known as farnesoid X receptor or NR1H4 is a nuclear receptor that is encoded by the NR1H4 gene in humans.- Function :...
, also known by its gene name . Activation of FXR in the liver inhibits synthesis of bile acids, and is one mechanism of feedback control when bile acid levels are too high. FXR activation by bile acids during absorption in the intestine increasees transcription and synthesis of FGF19
FGF19
Fibroblast growth factor 19 is a protein that in humans is encoded by the FGF19 gene.-Further reading:...
, which will then inhibit bile acid synthesis in the liver. Emerging evidence associates FXR activation with alterations in triglyceride
Triglyceride
A triglyceride is an ester derived from glycerol and three fatty acids. There are many triglycerides, depending on the oil source, some are highly unsaturated, some less so....
metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
, glucose metabolism, and liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
growth.
Clinical significance
Since bile acids are made from endogenous cholesterol, the enterohepatic circulation of bile acids may be disrupted to lower cholesterol. Bile acid sequestrantBile acid sequestrant
The bile acid sequestrants are a group of medications used to bind certain components of bile in the gastrointestinal tract. They disrupt the enterohepatic circulation of bile acids by sequestering them and preventing their reabsorption from the gut. In general, they are classified as hypolipidemic...
s bind bile acids in the gut, preventing reabsorption. In so doing, more endogenous cholesterol is shunted into the production of bile acids, thereby lowering cholesterol levels. The sequestered bile acids are then excreted in the feces.
Tests for bile acids are useful in both human and veterinary medicine, as they help to diagnose a number of conditions, including cholestasis
Cholestasis
In medicine, cholestasis is a condition where bile cannot flow from the liver to the duodenum. The two basic distinctions are an obstructive type of cholestasis where there is a mechanical blockage in the duct system such as can occur from a gallstone or malignancy and metabolic types of...
, portosystemic shunt
Portosystemic shunt
A portosystemic shunt , also known as a liver shunt, is a bypass of the liver by the body's circulatory system. It can be either a congenital or acquired condition....
, and hepatic microvascular dysplasia
Hepatic microvascular dysplasia
Hepatic microvascular dysplasia or Portal Atresia is a disorder where mixing of venous blood and arterial blood in the liver occurs at the microscopic level. It occurs most commonly in certain dog breeds such as the Cairn and Yorkshire terriers although any dog breed may be at risk....
.
Excess concentrations of bile acids in the colon are a cause of chronic diarrhea. This condition of bile acid malabsorption
Bile acid malabsorption
Bile acid malabsorption is a cause of chronic diarrhea. It can result from malabsorption secondary to gastro-intestinal disease or be a primary disorder. Treatment with bile acid sequestrants is often effective.-Classification:...
can be diagnosed by the SeHCAT
SeHCAT
SeHCAT is the usual name for 23-seleno-25-homo-tauro-cholic acid . It is used in a clinical test to diagnose bile acid malabsorption.-Development:...
test and treated with bile acid sequestrants .
The role of black bile in carcinogenesis was suggested already by Hippocrates. Today it's well-documented that bile acids are carcinogens and tumor promotors in experimental models. Their role in carcinogenesis is best documented in Barrett's esophagus
Barrett's esophagus
Barrett's esophagus refers to an abnormal change in the cells of the inferior portion of the esophagus. A positive diagnosis generally requires observing specific macroscopic and microscopic changes...
and adenocarcinoma at the gastroesophageal junctions.

