Chenodeoxycholic acid
Encyclopedia
Chenodeoxycholic acid is a bile acid
. It occurs as a white crystalline substance insoluble in water but soluble in alcohol
and acetic acid
, with melting point at 165-167 °C. Salts of this carboxylic acid
are called chenodeoxycholates. Chenodeoxycholic acid is one of the 4 main organic acid
s produced by the liver
.
Chenodeoxycholic acid is synthesized in the liver from cholesterol
. It was first isolated in the domestic goose, hence the 'cheno' portion of its name (Greek: χήνα = goose)
This compound, when altered by bacteria
in the colon
, will result in conversion to its secondary bile acid known as lithocholic acid
. Both of these bile acids, in addition to the others, can be conjugated to taurine or glycine
. Conjugation, a function carried out by the liver
will result in a lowered pKa and therefore, the compounds will remain ionized. These ionized compounds will stay in the gastrointestinal tract until reaching the ileum
where they will be reabsorbed. The purpose of this conjugation is to keep the bile acids in the tract until the end to facilitate lipid digestion all the way to the ileum.
In cases where bacteria overgrow in the small intestine, often due to a blind loop in the intestine retaining chyme in one place, the bacteria will de-conjugate the bile acids and therefore impede fat digestion and absorption. This can lead to steatorrhea
.
Chenodeoxycholic acid and cholic acid
are the most important human bile acids. Some other mammals synthesize predominantly deoxycholic acid
.
has developed a treatment for Hepatitis C
infection that combines chenodeoxycholic acid with bezafibrate
.
Chenodeoxycholic acid can be used in the treatment of cerebrotendinous xanthomatosis.
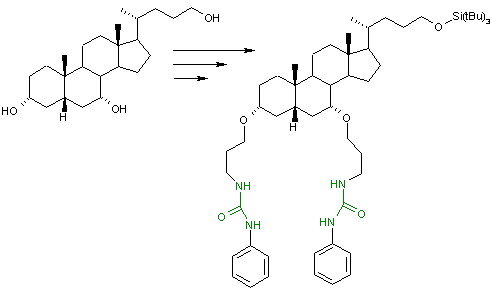
In supramolecular chemistry
, molecular tweezers based on a chenodeoxycholic acid scaffold is a urea
receptor that can contain anions in its binding pocket in order of affinity: H2PO4- (dihydrogen phosphate
) > Cl
- > Br
- > I
- reflecting their basicities
(tetrabutylammonium counter ion).
Bile acid
Bile acids are steroid acids found predominantly in the bile of mammals. Bile salts are bile acids compounded with a cation, usually sodium. In humans, the salts of taurocholic acid and glycocholic acid represent approximately eighty percent of all bile salts. The two major bile acids are cholic...
. It occurs as a white crystalline substance insoluble in water but soluble in alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
and acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
, with melting point at 165-167 °C. Salts of this carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
are called chenodeoxycholates. Chenodeoxycholic acid is one of the 4 main organic acid
Organic acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. The relative stability of the conjugate...
s produced by the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
.
Chenodeoxycholic acid is synthesized in the liver from cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
. It was first isolated in the domestic goose, hence the 'cheno' portion of its name (Greek: χήνα = goose)
This compound, when altered by bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
in the colon
Colon (anatomy)
The colon is the last part of the digestive system in most vertebrates; it extracts water and salt from solid wastes before they are eliminated from the body, and is the site in which flora-aided fermentation of unabsorbed material occurs. Unlike the small intestine, the colon does not play a...
, will result in conversion to its secondary bile acid known as lithocholic acid
Lithocholic acid
Lithocholic acid is a bile acid that acts as a detergent to solubilize fats for absorption. It is made from chenodeoxycholic acid by bacterial action in the colon.It has been implicated in human and experimental animal carcinogenesis....
. Both of these bile acids, in addition to the others, can be conjugated to taurine or glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
. Conjugation, a function carried out by the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
will result in a lowered pKa and therefore, the compounds will remain ionized. These ionized compounds will stay in the gastrointestinal tract until reaching the ileum
Ileum
The ileum is the final section of the small intestine in most higher vertebrates, including mammals, reptiles, and birds. In fish, the divisions of the small intestine are not as clear and the terms posterior intestine or distal intestine may be used instead of ileum.The ileum follows the duodenum...
where they will be reabsorbed. The purpose of this conjugation is to keep the bile acids in the tract until the end to facilitate lipid digestion all the way to the ileum.
In cases where bacteria overgrow in the small intestine, often due to a blind loop in the intestine retaining chyme in one place, the bacteria will de-conjugate the bile acids and therefore impede fat digestion and absorption. This can lead to steatorrhea
Steatorrhea
Steatorrhea is the presence of excess fat in feces. Stools may also float due to excess lipid, have an oily appearance and be especially foul-smelling. An oily anal leakage or some level of fecal incontinence may occur. There is increased fat excretion, which can be measured by determining the...
.
Chenodeoxycholic acid and cholic acid
Cholic acid
Cholic acid is a bile acid, a white crystalline substance insoluble in water , with a melting point of 200-201 °C. Salts of cholic acid are called cholates. Cholic acid, along with chenodeoxycholic acid, is one of two major bile acids produced by the liver where it is synthesized from cholesterol...
are the most important human bile acids. Some other mammals synthesize predominantly deoxycholic acid
Deoxycholic acid
Deoxycholic acid, also known as deoxycholate, cholanoic acid, and 3α,12α-dihydroxy-5β-cholanate, is a bile acid. Deoxycholic acid is one of the secondary bile acids, which are metabolic byproducts of intestinal bacteria. The two primary bile acids secreted by the liver are cholic acid and...
.
Potential applications
The Australian biotechnology company GiacondaGiaconda
Giaconda is an Australian biotechnology company headquartered in Sydney. The company was founded in 2004 to commercialise a number of drug combinations developed by Professor Thomas Borody, a Sydney-based gastroenterologist.-History:...
has developed a treatment for Hepatitis C
Hepatitis C
Hepatitis C is an infectious disease primarily affecting the liver, caused by the hepatitis C virus . The infection is often asymptomatic, but chronic infection can lead to scarring of the liver and ultimately to cirrhosis, which is generally apparent after many years...
infection that combines chenodeoxycholic acid with bezafibrate
Bezafibrate
Bezafibrate is a fibrate drug used for the treatment of hyperlipidaemia. It helps to lower LDL cholesterol and triglyceride in the blood, and increase HDL.-Mode of action:...
.
Chenodeoxycholic acid can be used in the treatment of cerebrotendinous xanthomatosis.
In supramolecular chemistry
Supramolecular chemistry
Supramolecular chemistry refers to the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components...
, molecular tweezers based on a chenodeoxycholic acid scaffold is a urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
receptor that can contain anions in its binding pocket in order of affinity: H2PO4- (dihydrogen phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
) > Cl
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
- > Br
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
- > I
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
- reflecting their basicities
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
(tetrabutylammonium counter ion).