
Calmodulin
Encyclopedia
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
-binding protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
expressed in all eukaryotic cells
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
. It can bind to and regulate a number of different protein targets, thereby affecting many different cellular functions.
Function
CaM mediates processes such as inflammationInflammation
Inflammation is part of the complex biological response of vascular tissues to harmful stimuli, such as pathogens, damaged cells, or irritants. Inflammation is a protective attempt by the organism to remove the injurious stimuli and to initiate the healing process...
, metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
, apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
, smooth muscle
Smooth muscle
Smooth muscle is an involuntary non-striated muscle. It is divided into two sub-groups; the single-unit and multiunit smooth muscle. Within single-unit smooth muscle tissues, the autonomic nervous system innervates a single cell within a sheet or bundle and the action potential is propagated by...
contraction, intracellular movement, short-term
Short-term memory
Short-term memory is the capacity for holding a small amount of information in mind in an active, readily available state for a short period of time. The duration of short-term memory is believed to be in the order of seconds. A commonly cited capacity is 7 ± 2 elements...
and long-term memory
Long-term memory
Long-term memory is memory in which associations among items are stored, as part of the theory of a dual-store memory model. According to the theory, long term memory differs structurally and functionally from working memory or short-term memory, which ostensibly stores items for only around 20–30...
, nerve growth and the immune response. CaM is expressed in many cell types and can have different subcellular locations, including the cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
, within organelle
Organelle
In cell biology, an organelle is a specialized subunit within a cell that has a specific function, and is usually separately enclosed within its own lipid bilayer....
s, or associated with the plasma or organelle membranes. Many of the proteins that CaM binds are unable to bind calcium themselves, and as such use CaM as a calcium sensor and signal transducer. CaM can also make use of the calcium stores in the endoplasmic reticulum
Endoplasmic reticulum
The endoplasmic reticulum is an organelle of cells in eukaryotic organisms that forms an interconnected network of tubules, vesicles, and cisternae...
, and the sarcoplasmic reticulum. CaM undergoes a conformational change upon binding to calcium, which enables it to bind to specific proteins for a specific response. CaM can bind up to four calcium ions, and can undergo post-translational modifications, such as phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
, acetylation
Acetylation
Acetylation describes a reaction that introduces an acetyl functional group into a chemical compound...
, methylation
Methylation
In the chemical sciences, methylation denotes the addition of a methyl group to a substrate or the substitution of an atom or group by a methyl group. Methylation is a form of alkylation with, to be specific, a methyl group, rather than a larger carbon chain, replacing a hydrogen atom...
and proteolytic cleavage, each of which has potential to modulate its actions. Calmodulin can also bind to edema factor toxin
Anthrax toxin
Anthrax toxin is a three-protein exotoxin secreted by virulent strains of the bacterium, Bacillus anthracis--the causative agent of anthrax. The toxin was first discovered by Harry Smith in 1954. Anthrax toxin is composed of a cell-binding protein, known as protective antigen , and two enzyme...
from the anthrax
Anthrax
Anthrax is an acute disease caused by the bacterium Bacillus anthracis. Most forms of the disease are lethal, and it affects both humans and other animals...
bacteria.
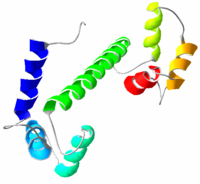
Structure
Calmodulin is a small, acidAcid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic protein approximately 148 amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s long (16706 Dalton
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
) and, as such, is a favorite for testing protein simulation software
Computational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
. It contains four EF-hand
EF hand
The EF hand is a helix-loop-helix structural domain found in a large family of calcium-binding proteins. The EF-hand motif contains a helix-loop-helix topology, much like the spread thumb and forefinger of the human hand, in which the Ca2+ ions are coordinated by ligands within the loop...
"motifs
Structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
", each of which binds a Ca2+ ion. The protein has two approximately symmetrical domains, separated by a flexible "hinge" region. Calcium participates in an intracellular signalling system by acting as a diffusible second messenger to the initial stimuli.
Mechanism
Calcium is bound via the use of the EF hand motif, which supplies an electronegative environment for ionIon
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
coordination. After calcium binding, hydrophobic methyl groups from methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
residues become exposed on the protein via conformational change
Conformational change
A macromolecule is usually flexible and dynamic. It can change its shape in response to changes in its environment or other factors; each possible shape is called a conformation, and a transition between them is called a conformational change...
. This presents hydrophobic surfaces, which can in turn bind to Basic Amphiphilic Helices (BAA helices) on the target protein. These helices contain complementary hydrophobic regions. The flexibility of Calmodulin's hinged region allows the molecule to "wrap around" its target. This property allows it to tightly bind to a wide range of different target proteins.
Dynamic features
Compared to the X-ray crystal structure, the C-terminal domain solution structureProtein nuclear magnetic resonance spectroscopy
Nuclear magnetic resonance spectroscopy of proteins is a field of structural biology in which NMR spectroscopy is used to obtain information about the structure and dynamics of proteins. The field was pioneered by Richard R. Ernst and Kurt Wüthrich, among others...
is similar while the EF hand
EF hand
The EF hand is a helix-loop-helix structural domain found in a large family of calcium-binding proteins. The EF-hand motif contains a helix-loop-helix topology, much like the spread thumb and forefinger of the human hand, in which the Ca2+ ions are coordinated by ligands within the loop...
s of the N-terminal domain are considerably less open. The backbone flexibility within calmodulin is key to its ability to bind a wide range of targets.
Family members
- Calmodulin 1Calmodulin 1Calmodulin 1 is a protein that in humans is encoded by the CALM1 gene.- Function :Calmodulin 1 is the archetype of the family of calcium-modulated proteins of which nearly 20 members have been found. They are identified by their occurrence in the cytosol or on membranes facing the cytosol and by a...
- Calmodulin 2CALM2Calmodulin 2 is a protein that in humans is encoded by the CALM2 gene.- Further reading :...
- Calmodulin 3CALM3Calmodulin is a protein that in humans is encoded by the CALM3 gene.-Further reading:...
- Calmodulin-like 1
- Calmodulin-like 3CALML3Calmodulin-like protein 3 is a protein that in humans is encoded by the CALML3 gene.-Further reading:...
- Calmodulin-like 4
- Calmodulin-like 5CALML5Calmodulin-like protein 5 is a protein that in humans is encoded by the CALML5 gene.-Further reading:...
- Calmodulin-like 6
Other calcium-binding proteins
Calmodulin belongs to one of the two main groups of calcium-binding proteins, called EF hand proteins. The other group, called annexinAnnexin
Annexin is a common name for a group of cellular proteins. They are found in all kingdoms with the exception of the bacteria....
s, bind calcium and phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
(e.g., lipocortin). Many other proteins bind calcium, although binding calcium may not be considered their principal function in the cell.
See also
- Proteopedia page for Calmodulin and bird its conformational change
- Protein kinaseProtein kinaseA protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them . Phosphorylation usually results in a functional change of the target protein by changing enzyme activity, cellular location, or association with other proteins...
- Ca2+/calmodulin-dependent protein kinaseCa2+/calmodulin-dependent protein kinase/calmodulin-dependent protein kinases II or CaM kinases II are serine/threonine-specific protein kinases that are regulated by the /calmodulin complex...

