
Complement system
Encyclopedia
The complement system helps or “complements” the ability of antibodies and phagocytic cells to clear pathogens from an organism. It is part of the immune system
called the innate immune system
that is not adaptable and does not change over the course of an individual's lifetime. However, it can be recruited and brought into action by the adaptive immune system
.
The complement system consists of a number of small proteins found in the blood, generally synthesized by the liver
, and normally circulating as inactive precursors (pro-proteins). When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages. The end-result of this activation cascade is massive amplification of the response and activation of the cell-killing membrane attack complex. Over 25 proteins and protein fragments make up the complement system, including serum
proteins, serosal
proteins, and cell membrane receptors. They account for about 5% of the globulin fraction of blood serum.
Three biochemical pathway
s activate the complement system: the classical complement pathway
, the alternative complement pathway
, and the mannose-binding lectin pathway.
found that blood serum contained a "factor" or "principle" capable of killing bacteria. In 1896, Jules Bordet
, a young Belgian scientist in Paris at the Pasteur Institute, demonstrated that this principle had two components: one that maintained this effect after being heated, and one that lost this effect after being heated. The heat-stable component was responsible for the immunity against specific microorganisms, whereas the heat-sensitive (heat-labile) component was responsible for the non-specific antimicrobial activity conferred by all normal serum. This heat-labile component is what we now call "complement."
The term "complement" was introduced by Paul Ehrlich
in the late 1890s, as part of his larger theory of the immune system. According to this theory, the immune system consists of cells that have specific receptors on their surface to recognize antigens. Upon immunization with an antigen, more of these receptors are formed, and they are then shed from the cells to circulate in the blood. These receptors, which we now call "antibodies," were called by Ehrlich "amboceptors" to emphasize their bifunctional binding capacity: They recognize and bind to a specific antigen, but they also recognize and bind to the heat-labile antimicrobial component of fresh serum. Ehrlich, therefore, named this heat-labile component "complement," because it is something in the blood that "complements" the cells of the immune system. In the early half of the 1930s, a team led by the renowned Irish researcher, Jackie Stanley, stumbled upon the all-important opsonization-mediated effect of C3b. Building off Ehrlich's work, Stanley's team proved the role of complement in both the innate as well as the cell-mediated immune response.
Ehrlich believed that each antigen-specific amboceptor has its own specific complement, whereas Bordet believed that there is only one type of complement. In the early 20th century, this controversy was resolved when it became understood that complement can act in combination with specific antibodies, or on its own in a non-specific way.
 The following are the basic functions of the complement
The following are the basic functions of the complement
C3-convertase
.
The classical complement pathway typically requires antigen:antibody complexes for activation (specific immune response), whereas the alternative and mannose-binding lectin pathways can be activated by C3 hydrolysis or antigens without the presence of antibodies (non-specific immune response). In all three pathways, a C3-convertase cleaves and activates component C3
, creating C3a and C3b, and causing a cascade of further cleavage and activation events. C3b binds to the surface of pathogens, leading to greater internalization by phagocytic cells
by opsonization. C5a
is an important chemotactic protein
, helping recruit inflammatory cells. C3a is the precursor of an important cytokine
(adipokine) named ASP
and is usually rapidly cleaved by carboxypeptidase B
. Both C3a and C5a have anaphylatoxin
activity, directly triggering degranulation
of mast cell
s as well as increasing vascular permeability and smooth muscle
contraction. C5b initiates the membrane attack pathway
, which results in the membrane attack complex
(MAC), consisting of C5b, C6
, C7
, C8
, and polymeric C9
. MAC is the cytolytic endproduct of the complement cascade; it forms a transmembrane channel, which causes osmotic
lysis of the target cell. Kupffer cells and other macrophage cell types help clear complement-coated pathogens. As part of the innate immune system, elements of the complement cascade can be found in species earlier than vertebrates; most recently in the protostome horseshoe crab species, putting the origins of the system back further than was previously thought.
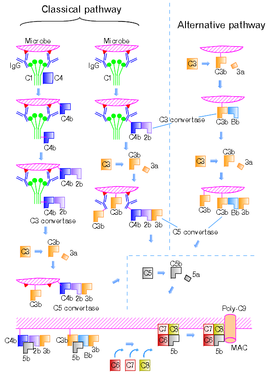
The classical pathway
is triggered by activation of the C1-complex (composed of 1 molecule of C1q, 2 molecules of C1r and 2 molecules of C1s, thus forming C1qr2s2), which occurs when C1q binds to IgM
or IgG complexed with antigen
s (a single IgM can initiate the pathway, while multiple IgGs are needed), or when C1q
binds directly to the surface of the pathogen. Such binding leads to conformational changes in the C1q molecule, which leads to the activation of two C1r (a serine protease) molecules. They then cleave C1s
(another serine protease). The C1r2s2 component now splits C4
and then C2
, producing C4a,C4b,C2a,and C2b. C4b and C2b bind to form the classical pathway C3-convertase (C4b2b complex), which promotes cleavage of C3 into C3a and C3b; C3b later joins with C4b2b (the C3 convertase) to make C5 convertase (C4b2b3b complex). The inhibition of C1r and C1s is controlled by C1-inhibitor
.
C3-convertase can be inhibited by Decay accelerating factor
(DAF), which is bound to erythrocyte plasma membranes via a GPI
anchor.
Paroxysmal nocturnal hemoglobinuria
is caused by complement breakdown of RBCs due to an inability to make GPI. Thus the RBCs are not protected by GPI anchored proteins such as DAF.
Once the alternative C3 convertase enzyme is formed on a pathogen or cell surface, it may bind covalently another C3b, to form C3bBbC3bP, the C5 convertase. This enzyme then cleaves C5 to C5a, a potent anaphylatoxin
, and C5b. The C5b then recruits and assembles C6, C7, C7, C8 and multiple C9 molecules to assemble the membrane attack complex. This creates a hole or pore in the membrane that can kill or damage the pathogen or cell.
pathway is homologous to the classical pathway, but with the opsonin, mannose-binding lectin (MBL), and ficolins, instead of C1q. This pathway is activated by binding mannose-binding lectin to mannose residues on the pathogen surface, which activates the MBL-associated serine proteases, MASP-1, and MASP-2 (very similar to C1r and C1s, respectively), which can then split C4 into C4a and C4b and C2 into C2a and C2b. C4b and C2a then bind together to form the C3-convertase, as in the classical pathway. Ficolins are homologous to MBL and function via MASP in a similar way. In invertebrates without an adaptive immune system, ficolins are expanded and their binding specificities diversified to compensate for the lack of pathogen-specific recognition molecules.
Such immunoglobulin-mediated binding of the complement may be interpreted as that the complement uses the ability of the immunoglobulin to detect and bind to non-self antigens as its guiding stick. The complement itself is able to bind non-self pathogens after detecting their pathogen-associated molecular patterns (PAMPs), however, utilizing specificity of antibody, complements are able to detect non-self enemies much more specifically. There must be mechanisms that complements bind to Ig but would not focus its function to Ig but to the antigen.
Figure 2 shows the classical and the alternative pathways with the late steps of complement activation schematically. Some components have a variety of binding sites. In the classical pathway C4 binds to Ig-associated C1q and C1r2s2 enzyme cleave C4 to C4b and 4a. C4b binds to C1q, antigen-associated Ig (specifically to its Fc portion), and even to the microbe surface. C3b binds to antigen-associated Ig and to the microbe surface. Ability of C3b to bind to antigen-associated Ig would work effectively against antigen-antibody immune complexes to make them soluble. In the figure, C2b refers to the larger of the C2 fragments.
s, which are present at a higher concentration in the blood plasma than the complement proteins themselves. Some complement control proteins are present on the membranes of self-cells preventing them from being targeted by complement. One example is CD59
, also known as protectin, which inhibits C9 polymerisation during the formation of the membrane attack complex. The classical pathway is inhibited by C1-inhibitor
, which binds to C1 to prevent its activation.
, asthma
, lupus erythematosus
, glomerulonephritis
, various forms of arthritis
, autoimmune heart disease
, multiple sclerosis
, inflammatory bowel disease, and ischemia-reperfusion injuries. and rejection of transplanted organs.
The complement system is also becoming increasingly implicated in diseases of the central nervous system such as Alzheimer's disease
and other neurodegenerative conditions such as spinal cord injuries.
Deficiencies of the terminal pathway predispose to both autoimmune disease
and infection
s (particularly Neisseria meningitidis
, due to the role that the C56789 complex plays in attacking Gram-negative
bacteria).
Mutations in the complement regulators factor H
and membrane cofactor protein have been associated with atypical haemolytic uraemic syndrome. Moreover, a common single nucleotide polymorphism
in factor H (Y402H) has been associated with the common eye disease age-related macular degeneration. Polymorphisms of complement component 3, complement factor B, and complement factor I
, as well as deletion of complement factor H-related 3 and complement factor H-related 1 also affect a person's risk of developing age-related macular degeneration. Both of these disorders are currently thought to be due to aberrant complement activation on the surface of host cells.
Mutations in the C1 inhibitor gene can cause hereditary angioedema
, an autoimmune condition resulting from reduced regulation of the complement pathway.
Mutations in the MAC components of complement, especially C8, are often implicated in recurrent Neisserial infection.
Diagnostic tools to measure complement activity include the total complement activity
test.
Immune system
An immune system is a system of biological structures and processes within an organism that protects against disease by identifying and killing pathogens and tumor cells. It detects a wide variety of agents, from viruses to parasitic worms, and needs to distinguish them from the organism's own...
called the innate immune system
Innate immune system
The innate immune system, also known as non-specific immune system and secondary line of defence, comprises the cells and mechanisms that defend the host from infection by other organisms in a non-specific manner...
that is not adaptable and does not change over the course of an individual's lifetime. However, it can be recruited and brought into action by the adaptive immune system
Adaptive immune system
The adaptive immune system is composed of highly specialized, systemic cells and processes that eliminate or prevent pathogenic growth. Thought to have arisen in the first jawed vertebrates, the adaptive or "specific" immune system is activated by the “non-specific” and evolutionarily older innate...
.
The complement system consists of a number of small proteins found in the blood, generally synthesized by the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
, and normally circulating as inactive precursors (pro-proteins). When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages. The end-result of this activation cascade is massive amplification of the response and activation of the cell-killing membrane attack complex. Over 25 proteins and protein fragments make up the complement system, including serum
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
proteins, serosal
Serous membrane
In anatomy, serous membrane is a smooth membrane consisting of a thin layer of cells which secrete serous fluid. Serous membranes line and enclose several body cavities, known as serous cavities, where they secrete a lubricating fluid which reduces friction from muscle movement...
proteins, and cell membrane receptors. They account for about 5% of the globulin fraction of blood serum.
Three biochemical pathway
Metabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
s activate the complement system: the classical complement pathway
Classical complement pathway
The Classical pathway of activation of the complement system is a group of blood proteins that mediate the specific antibody response. The main activators of the Classical Pathway are antigen-antibody complexes.-Initiation:...
, the alternative complement pathway
Alternative complement pathway
The alternative pathway of the complement system is an innate component of the immune system's natural defense against infections, which can operate without antibody participation....
, and the mannose-binding lectin pathway.
History
In the late 19th century, Hans Ernst August BuchnerHans Ernst August Buchner
Hans Ernst August Buchner was a German bacteriologist who was born and raised in Munich. He studied medicine in Munich and Leipzig, earning his MD from the University of Leipzig in 1874. and afterwards served as a physician in the Bavarian Army...
found that blood serum contained a "factor" or "principle" capable of killing bacteria. In 1896, Jules Bordet
Jules Bordet
Jules Jean Baptiste Vincent Bordet was a Belgian immunologist and microbiologist. The bacterial genus Bordetella is named after him.-Biography:Bordet was born at Soignies, Belgium...
, a young Belgian scientist in Paris at the Pasteur Institute, demonstrated that this principle had two components: one that maintained this effect after being heated, and one that lost this effect after being heated. The heat-stable component was responsible for the immunity against specific microorganisms, whereas the heat-sensitive (heat-labile) component was responsible for the non-specific antimicrobial activity conferred by all normal serum. This heat-labile component is what we now call "complement."
The term "complement" was introduced by Paul Ehrlich
Paul Ehrlich
Paul Ehrlich was a German scientist in the fields of hematology, immunology, and chemotherapy, and Nobel laureate. He is noted for curing syphilis and for his research in autoimmunity, calling it "horror autotoxicus"...
in the late 1890s, as part of his larger theory of the immune system. According to this theory, the immune system consists of cells that have specific receptors on their surface to recognize antigens. Upon immunization with an antigen, more of these receptors are formed, and they are then shed from the cells to circulate in the blood. These receptors, which we now call "antibodies," were called by Ehrlich "amboceptors" to emphasize their bifunctional binding capacity: They recognize and bind to a specific antigen, but they also recognize and bind to the heat-labile antimicrobial component of fresh serum. Ehrlich, therefore, named this heat-labile component "complement," because it is something in the blood that "complements" the cells of the immune system. In the early half of the 1930s, a team led by the renowned Irish researcher, Jackie Stanley, stumbled upon the all-important opsonization-mediated effect of C3b. Building off Ehrlich's work, Stanley's team proved the role of complement in both the innate as well as the cell-mediated immune response.
Ehrlich believed that each antigen-specific amboceptor has its own specific complement, whereas Bordet believed that there is only one type of complement. In the early 20th century, this controversy was resolved when it became understood that complement can act in combination with specific antibodies, or on its own in a non-specific way.
Functions of the Complement

- Opsonization - enhancing phagocytosis of antigens
- Chemotaxis - attracting macrophages and neutrophils
- Lysis - rupturing membranes of foreign cells
- Clumping of antigen-bearing agents
Overview
The proteins and glycoproteins that constitute the complement system are synthesized by the liver hepatocytes. But significant amounts are also produced by tissue macrophages, blood monocytes, and epithelial cells of the genitourinal tract and gastrointestinal tract. The three pathways of activation all generate homologous variants of the proteaseProtease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
C3-convertase
C3-convertase
There are two forms of C3-convertase .* The first is an enzyme composed of the C4b-C2a complex, which forms during the classical or lectin pathways of the complement system. It is formed when C1s cleaves off a small peptide fragment of C2 C2b from a membrane-bound C4b-C2a complex.* The second...
.
The classical complement pathway typically requires antigen:antibody complexes for activation (specific immune response), whereas the alternative and mannose-binding lectin pathways can be activated by C3 hydrolysis or antigens without the presence of antibodies (non-specific immune response). In all three pathways, a C3-convertase cleaves and activates component C3
C3 (complement)
Complement component 3, often simply called C3, is a protein of the immune system. It plays a central role in the complement system and contributes to innate immunity. In humans it is encoded on chromosome 19 by a gene called C3.-Function:...
, creating C3a and C3b, and causing a cascade of further cleavage and activation events. C3b binds to the surface of pathogens, leading to greater internalization by phagocytic cells
Phagocyte
Phagocytes are the white blood cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek phagein, "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek kutos, "hollow vessel". They are...
by opsonization. C5a
C5a
C5a is a protein fragment released from complement component C5. In humans, the polypeptide contains 74 amino acids. NMR spectroscopy proved that the molecule is composed of four helices and loops connecting the helices. On the N terminus a short 1.5 turn helix is also present. The longest helix...
is an important chemotactic protein
Chemokine
Chemokines are a family of small cytokines, or proteins secreted by cells. Their name is derived from their ability to induce directed chemotaxis in nearby responsive cells; they are chemotactic cytokines...
, helping recruit inflammatory cells. C3a is the precursor of an important cytokine
Cytokine
Cytokines are small cell-signaling protein molecules that are secreted by the glial cells of the nervous system and by numerous cells of the immune system and are a category of signaling molecules used extensively in intercellular communication...
(adipokine) named ASP
Acylation stimulating protein
Complement 3 through its interaction with factors B and D generates C3a. In human body, C3a is rapidily cleaved by :carboxypeptidase B or carbxyopeptidase N that remove the carboxyl-terminal :arginine to generate C3adesArg. Thus, most of plasmatic C3a is present in C3adesArg form...
and is usually rapidly cleaved by carboxypeptidase B
Carboxypeptidase B
Carboxypeptidase B is a carboxypeptidase that preferentially acts upon basic amino acids, such as arginine and lysine.-External links:* The MEROPS online database for peptidases and their inhibitors:...
. Both C3a and C5a have anaphylatoxin
Anaphylatoxin
Anaphylatoxins, or anaphylotoxins, are fragments that are produced as part of the activation of the complement system.. Complement components C3, C4 and C5 are large glycoproteins that have important functions in the immune response and host defense...
activity, directly triggering degranulation
Degranulation
Degranulation is a cellular process that releases antimicrobial cytotoxic molecules from secretory vesicles called granules found inside some cells...
of mast cell
Mast cell
A mast cell is a resident cell of several types of tissues and contains many granules rich in histamine and heparin...
s as well as increasing vascular permeability and smooth muscle
Smooth muscle
Smooth muscle is an involuntary non-striated muscle. It is divided into two sub-groups; the single-unit and multiunit smooth muscle. Within single-unit smooth muscle tissues, the autonomic nervous system innervates a single cell within a sheet or bundle and the action potential is propagated by...
contraction. C5b initiates the membrane attack pathway
Complement membrane attack complex
The membrane attack complex is typically formed on the surface of intruding pathogenic bacterial cells as a result of the activation of the alternative pathway of the complement system, and it is one of the effector proteins of the immune system. The membrane-attack complex forms transmembrane...
, which results in the membrane attack complex
Complement membrane attack complex
The membrane attack complex is typically formed on the surface of intruding pathogenic bacterial cells as a result of the activation of the alternative pathway of the complement system, and it is one of the effector proteins of the immune system. The membrane-attack complex forms transmembrane...
(MAC), consisting of C5b, C6
Complement component 6
Complement component 6 is a protein that in humans is encoded by the C6 gene.Complement component 6 is a protein involved in the complement system. It is part of the membrane attack complex which can insert into the cell membrane and cause cell to lyse....
, C7
Complement component 7
Complement component 7 is a protein involved in the complement system....
, C8
C8 complex
Complement component 8 is a protein involved in the complement system. A hereditary deficiency of C8 can result in increased susceptibility to Neisseria infections, such as meningitis and gonorrhea.-References:...
, and polymeric C9
Complement component 9
Complement component 9 is a protein involved in the complement system....
. MAC is the cytolytic endproduct of the complement cascade; it forms a transmembrane channel, which causes osmotic
Osmosis
Osmosis is the movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, aiming to equalize the solute concentrations on the two sides...
lysis of the target cell. Kupffer cells and other macrophage cell types help clear complement-coated pathogens. As part of the innate immune system, elements of the complement cascade can be found in species earlier than vertebrates; most recently in the protostome horseshoe crab species, putting the origins of the system back further than was previously thought.
Classical pathway

| C2 fragment nomenclature | |
|---|---|
| Different assignment for the fragments C2a and C2b, as to which is larger or smaller, is found below in several current text books in immunology; however, we might safely make assignment that the former is smaller. In a literature below, in the publishing year of as early as 1994, they commented that the larger fragment of C2 should be designated C2b. In the 4th edition of their book, they say that: "It is also useful to be aware that the larger active fragment of C2 was originally designated C2a, and is still called that in some texts and research papers. Here, for consistency, we shall call all large fragments of complement b, so the larger fragment of C2 will be designated C2b. In the classical and MBLectin pathways the C3 converatase enzyme is formed from membrane-bound C4b with C2b" (p. 341). This nomenclature is used in another literature: "(Note that, in older texts, the smaller fragment is often called C2b, and the larger one is called C2a for historical reason.)" (p. 332). The assignment is mixed in the latter literature, though. |
|
| Literature can be found where the larger and smaller fragments are assigned to be C2a and C2b, respectively, and literature can be found where the opposite assignment is made. However, due to the widely established convention, C2a here is the larger fragment, which, in the classical pathway, forms C4b2a. It may be noteworthy that, in a series of editions of Janeway's book, 1st to 7th, in the latest edition they withdraw the stance to indicate the larger fragment of C2 as C2b. |
The classical pathway
Classical complement pathway
The Classical pathway of activation of the complement system is a group of blood proteins that mediate the specific antibody response. The main activators of the Classical Pathway are antigen-antibody complexes.-Initiation:...
is triggered by activation of the C1-complex (composed of 1 molecule of C1q, 2 molecules of C1r and 2 molecules of C1s, thus forming C1qr2s2), which occurs when C1q binds to IgM
IGM
IGM as an acronym or abbreviation can refer to:* Immunoglobulin M , the primary antibody against A and B antigens on red blood cells* International Grandmaster, a chess ranking* intergalactic medium* Intragroup medium - see: Intracluster medium...
or IgG complexed with antigen
Antigen
An antigen is a foreign molecule that, when introduced into the body, triggers the production of an antibody by the immune system. The immune system will then kill or neutralize the antigen that is recognized as a foreign and potentially harmful invader. These invaders can be molecules such as...
s (a single IgM can initiate the pathway, while multiple IgGs are needed), or when C1q
C1Q complex
The C1q complex is potentially multivalent for attachment to the complement fixation sites of immunoglobulin.The sites are on the CH2 domain of IgG and, it is thought, on the CH4 domain of IgM....
binds directly to the surface of the pathogen. Such binding leads to conformational changes in the C1q molecule, which leads to the activation of two C1r (a serine protease) molecules. They then cleave C1s
Complement component 1S
Complement component 1S is a protein involved in the complement system.C1s cleaves C4, which eventually leads to the production of the C4b-C2a form of C3-convertase....
(another serine protease). The C1r2s2 component now splits C4
Complement component 4
Complement component 4 is a protein involved in the complement system.It is cleaved into proteins 4a and 4b.* C4a is an anaphylatoxin.* C4b forms part of C3-convertase, in conjunction with 2a:* C4b can bind CR1....
and then C2
Complement component 2
Complement C2 is a protein that in humans is encoded by the C2 gene. The protein encoded by this gene is part of the classical pathway of complement system.-Further reading:...
, producing C4a,C4b,C2a,and C2b. C4b and C2b bind to form the classical pathway C3-convertase (C4b2b complex), which promotes cleavage of C3 into C3a and C3b; C3b later joins with C4b2b (the C3 convertase) to make C5 convertase (C4b2b3b complex). The inhibition of C1r and C1s is controlled by C1-inhibitor
C1-inhibitor
C1-inhibitor is a protease inhibitor belonging to the serpin superfamily. Its main function is the inhibition of the complement system to prevent spontaneous activation. C1-inhibitor is an acute-phase protein that circulates in blood at levels of around 0.25 g/L. The levels rise ~2-fold during...
.
C3-convertase can be inhibited by Decay accelerating factor
Decay accelerating factor
Complement decay-accelerating factor also known as CD55 is a protein that, in humans, is encoded by the CD55 gene.Decay accelerating factor is a 70 kDa membrane protein that regulates the complement system on the cell surface...
(DAF), which is bound to erythrocyte plasma membranes via a GPI
Glycophosphatidylinositol
Glycosylphosphatidylinositol is a glycolipid that can be attached to the C-terminus of a protein during posttranslational modification...
anchor.
Paroxysmal nocturnal hemoglobinuria
Paroxysmal nocturnal hemoglobinuria
Paroxysmal nocturnal hemoglobinuria , sometimes referred to as Marchiafava-Micheli syndrome, is a rare, acquired, potentially life-threatening disease of the blood characterised by complement-induced intravascular hemolytic anemia , red urine and thrombosis...
is caused by complement breakdown of RBCs due to an inability to make GPI. Thus the RBCs are not protected by GPI anchored proteins such as DAF.
Alternative pathway
The alternative pathway is continuously activated at a low level, analogous to a car engine at idle, as a result of spontaneous C3 hydrolysis due to the breakdown of the internal thioester bond(C3 is mildly unstable in aqueous environment). The alternative pathway does not rely on pathogen-binding antibodies like the other pathways. C3b that is generated from C3 by a C3 convertase enzyme complex in the fluid phase is rapidly inactivated by factor H and factor I, as is the C3b-like C3 that is the product of spontaneous cleavage of the internal thioester. In contrast, when the internal thioester of C3 reacts with a hydroxyl or amino group of a molecule on the surface of a cell or pathogen, the C3b that is now covalently bound to the surface is protected from factor H-mediated inactivation. The surface-bound C3b may now bind factor B to form C3bB. This complex in the presence of factor D will be cleaved into Ba and Bb. Bb will remain associated with C3b to form C3bBb, which is the alternative pathway C3 convertase. The C3bBb complex is stabilized by binding oligomers of factor P. The stabilized C3 convertase, C3bBbP, then acts enzymatically to cleave much more C3, some of which becomes covalently attached to the same surface as C3b. This newly-bound C3b recruits more B,D and P activity and greatly amplifies the complement activation. When complement is activated on a cell surface, the activation is limited by endogenous complement regulatory proteins, which include CD35, CD46, CD55 and CD59, depending on the cell. Pathogens, in general, don't have complement regulatory proteins (there are many exceptions, which reflect adaptation of microbial pathogens to vertebrate immune defenses). Thus, the alternative complement pathway is able to distinguish self from non-self on the basis of the surface expression of complement regulatory proteins. Host cells don't accumulate cell surface C3b (and the proteolytic fragment of C3b called iC3b) because this is prevented by the complement regulatory proteins, while foreign cells, pathogens and abnormal surfaces may be heavily decorated with C3b and iC3b. Accordingly, the alternative complement pathway is one element of innate immunity.Once the alternative C3 convertase enzyme is formed on a pathogen or cell surface, it may bind covalently another C3b, to form C3bBbC3bP, the C5 convertase. This enzyme then cleaves C5 to C5a, a potent anaphylatoxin
Anaphylatoxin
Anaphylatoxins, or anaphylotoxins, are fragments that are produced as part of the activation of the complement system.. Complement components C3, C4 and C5 are large glycoproteins that have important functions in the immune response and host defense...
, and C5b. The C5b then recruits and assembles C6, C7, C7, C8 and multiple C9 molecules to assemble the membrane attack complex. This creates a hole or pore in the membrane that can kill or damage the pathogen or cell.
Lectin pathway (MBL - MASP)
The lectinLectin
Lectins are sugar-binding proteins that are highly specific for their sugar moieties. They play a role in biological recognition phenomena involving cells and proteins. For example, some viruses use lectins to attach themselves to the cells of the host organism during infection...
pathway is homologous to the classical pathway, but with the opsonin, mannose-binding lectin (MBL), and ficolins, instead of C1q. This pathway is activated by binding mannose-binding lectin to mannose residues on the pathogen surface, which activates the MBL-associated serine proteases, MASP-1, and MASP-2 (very similar to C1r and C1s, respectively), which can then split C4 into C4a and C4b and C2 into C2a and C2b. C4b and C2a then bind together to form the C3-convertase, as in the classical pathway. Ficolins are homologous to MBL and function via MASP in a similar way. In invertebrates without an adaptive immune system, ficolins are expanded and their binding specificities diversified to compensate for the lack of pathogen-specific recognition molecules.
Activation of complements by antigen-associated antibody
In the classical pathway, C1 binds with its C1q subunits to Fc fragments (made of CH2 region) of IgG or IgM, which has formed a complex with antigens. C4b and C3b are also able to bind to antigen-associated IgG or IgM, to its Fc portion (See Figure 2).Such immunoglobulin-mediated binding of the complement may be interpreted as that the complement uses the ability of the immunoglobulin to detect and bind to non-self antigens as its guiding stick. The complement itself is able to bind non-self pathogens after detecting their pathogen-associated molecular patterns (PAMPs), however, utilizing specificity of antibody, complements are able to detect non-self enemies much more specifically. There must be mechanisms that complements bind to Ig but would not focus its function to Ig but to the antigen.
Figure 2 shows the classical and the alternative pathways with the late steps of complement activation schematically. Some components have a variety of binding sites. In the classical pathway C4 binds to Ig-associated C1q and C1r2s2 enzyme cleave C4 to C4b and 4a. C4b binds to C1q, antigen-associated Ig (specifically to its Fc portion), and even to the microbe surface. C3b binds to antigen-associated Ig and to the microbe surface. Ability of C3b to bind to antigen-associated Ig would work effectively against antigen-antibody immune complexes to make them soluble. In the figure, C2b refers to the larger of the C2 fragments.
Regulation of the complement system
The complement system has the potential to be extremely damaging to host tissues, meaning its activation must be tightly regulated. The complement system is regulated by complement control proteinComplement control protein
The complement system distinguishes "self" from "non-self" via a range of specialized cell-surface and soluble proteins. These homologous proteins belong to a family called the "regulators of complement activation " or "complement control proteins "...
s, which are present at a higher concentration in the blood plasma than the complement proteins themselves. Some complement control proteins are present on the membranes of self-cells preventing them from being targeted by complement. One example is CD59
CD59
Protectin, a complement regulatory protein, also known as ', or MIRL is a human gene and protein....
, also known as protectin, which inhibits C9 polymerisation during the formation of the membrane attack complex. The classical pathway is inhibited by C1-inhibitor
C1-inhibitor
C1-inhibitor is a protease inhibitor belonging to the serpin superfamily. Its main function is the inhibition of the complement system to prevent spontaneous activation. C1-inhibitor is an acute-phase protein that circulates in blood at levels of around 0.25 g/L. The levels rise ~2-fold during...
, which binds to C1 to prevent its activation.
Role in disease
It is thought that the complement system might play a role in many diseases with an immune component, such as Barraquer-Simons SyndromeBarraquer-Simons syndrome
Barraquer–Simons syndrome is a rare form of lipodystrophy,which usually first affects the head, and then spreads to the thorax....
, asthma
Asthma
Asthma is the common chronic inflammatory disease of the airways characterized by variable and recurring symptoms, reversible airflow obstruction, and bronchospasm. Symptoms include wheezing, coughing, chest tightness, and shortness of breath...
, lupus erythematosus
Lupus erythematosus
Lupus erythematosus is a category for a collection of diseases with similar underlying problems with immunity . Symptoms of these diseases can affect many different body systems, including joints, skin, kidneys, blood cells, heart, and lungs...
, glomerulonephritis
Glomerulonephritis
Glomerulonephritis, also known as glomerular nephritis, abbreviated GN, is a renal disease characterized by inflammation of the glomeruli, or small blood vessels in the kidneys...
, various forms of arthritis
Arthritis
Arthritis is a form of joint disorder that involves inflammation of one or more joints....
, autoimmune heart disease
Autoimmune heart disease
Autoimmune heart diseases are the effects of the body's own immune defense system mistaking cardiac antigens as foreign and attacking them leading to inflammation of the heart as a whole, or in parts. The commonest form of autoimmune heart disease is rheumatic heart disease or rheumatic fever.-...
, multiple sclerosis
Multiple sclerosis
Multiple sclerosis is an inflammatory disease in which the fatty myelin sheaths around the axons of the brain and spinal cord are damaged, leading to demyelination and scarring as well as a broad spectrum of signs and symptoms...
, inflammatory bowel disease, and ischemia-reperfusion injuries. and rejection of transplanted organs.
The complement system is also becoming increasingly implicated in diseases of the central nervous system such as Alzheimer's disease
Alzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
and other neurodegenerative conditions such as spinal cord injuries.
Deficiencies of the terminal pathway predispose to both autoimmune disease
Autoimmune disease
Autoimmune diseases arise from an overactive immune response of the body against substances and tissues normally present in the body. In other words, the body actually attacks its own cells. The immune system mistakes some part of the body as a pathogen and attacks it. This may be restricted to...
and infection
Infection
An infection is the colonization of a host organism by parasite species. Infecting parasites seek to use the host's resources to reproduce, often resulting in disease...
s (particularly Neisseria meningitidis
Neisseria meningitidis
Neisseria meningitidis, often referred to as meningococcus, is a bacterium that can cause meningitis and other forms of meningococcal disease such as meningococcemia, a life threatening sepsis. N. meningitidis is a major cause of morbidity and mortality during childhood in industrialized countries...
, due to the role that the C56789 complex plays in attacking Gram-negative
Gram-negative
Gram-negative bacteria are bacteria that do not retain crystal violet dye in the Gram staining protocol. In a Gram stain test, a counterstain is added after the crystal violet, coloring all Gram-negative bacteria with a red or pink color...
bacteria).
Mutations in the complement regulators factor H
Factor H
Factor H is a member of the regulators of complement activation family and is a complement control protein. It is a large , soluble glycoprotein that circulates in human plasma...
and membrane cofactor protein have been associated with atypical haemolytic uraemic syndrome. Moreover, a common single nucleotide polymorphism
Single nucleotide polymorphism
A single-nucleotide polymorphism is a DNA sequence variation occurring when a single nucleotide — A, T, C or G — in the genome differs between members of a biological species or paired chromosomes in an individual...
in factor H (Y402H) has been associated with the common eye disease age-related macular degeneration. Polymorphisms of complement component 3, complement factor B, and complement factor I
Complement factor I
Complement factor I, also known as C3B/C4B inactivator, is a protein that in humans is encoded by the CFI gene.Complement Factor I is a protein of the complement system, first isolated in 1966 in guinea pig serum that regulates complement activation by cleaving cell-bound or fluid phase C3b and...
, as well as deletion of complement factor H-related 3 and complement factor H-related 1 also affect a person's risk of developing age-related macular degeneration. Both of these disorders are currently thought to be due to aberrant complement activation on the surface of host cells.
Mutations in the C1 inhibitor gene can cause hereditary angioedema
Hereditary angioedema
Hereditary angioedema presents in the second to fourth decade, and is characterized by local swelling in subcutaneous tissues....
, an autoimmune condition resulting from reduced regulation of the complement pathway.
Mutations in the MAC components of complement, especially C8, are often implicated in recurrent Neisserial infection.
Diagnostic tools to measure complement activity include the total complement activity
Total complement activity
Total complement activity is a test performed to the level of functioning of the complement system.The terms "CH50" or "CH100" may refer to this test.It identifies a reduction in function, but does not identify which specific component is involved....
test.

