Cyclic compound
Encyclopedia
In chemistry
, a cyclic compound is a compound
in which a series of atoms is connected to form a loop or ring.
While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. Cyclic compounds may or may not be aromatic
. Benzene
is a well known example. The term "polycyclic" is used when more than one ring is formed in a single molecule for instance in naphthalene
, and the term macrocycle
is used for a ring containing more than a dozen atoms.
Alicyclic compound are named according to the IUPAC system of nomenclature by attaching the prefix cyclo- to the name of the corresponding open chain hydrocarbon possessing the same number of carbon atoms. The common names resemble the IUPAC names. For example: Cyclo pantane, cyclo butane etc....
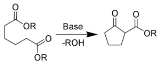
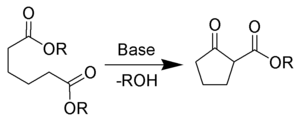 Related concepts in organic chemistry are so-called ring-closing reactions in which a cyclic compound is formed and ring-opening reactions in which rings are opened.
Related concepts in organic chemistry are so-called ring-closing reactions in which a cyclic compound is formed and ring-opening reactions in which rings are opened.
Examples of ring-closing reactions:
Example of ring-opening reactions:
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a cyclic compound is a compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
in which a series of atoms is connected to form a loop or ring.
While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. Cyclic compounds may or may not be aromatic
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
. Benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
is a well known example. The term "polycyclic" is used when more than one ring is formed in a single molecule for instance in naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
, and the term macrocycle
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
is used for a ring containing more than a dozen atoms.
Alicyclic compound are named according to the IUPAC system of nomenclature by attaching the prefix cyclo- to the name of the corresponding open chain hydrocarbon possessing the same number of carbon atoms. The common names resemble the IUPAC names. For example: Cyclo pantane, cyclo butane etc....
Ring-closing & opening reactions

Examples of ring-closing reactions:
- Ring-closing metathesisRing-closing metathesisRing-closing metathesis or RCM is a variation on olefin metathesis that allows the closing of previously hard to make rings...
- Nazarov cyclization reactionNazarov cyclization reactionThe Nazarov cyclization reaction is a chemical reaction used in organic chemistry for the synthesis of cyclopentenones. The reaction is typically divided into classical and modern variants, depending on the reagents and substrates employed...
- Ruzicka large ring synthesisRuzicka large ring synthesisThe Ruzicka large ring synthesis or Ruzicka reaction or Ruzicka cyclization is an organic reaction in which a dicarboxylic acid is converted to a cyclic ketone at high temperature and a suitable catalyst such as thorium oxide...
- Dieckmann condensationDieckmann condensationThe Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-ketoesters. It is named after the German chemist Walter Dieckmann . The equivalent intermolecular reaction is the Claisen condensation....
- Wenker synthesisWenker synthesisThe Wenker synthesis is an organic reaction converting a beta amino alcohol to an aziridine with the aid of sulfuric acid.The original Wenker synthesis of aziridine itself takes place in two steps...
- Radical cyclizationRadical cyclizationRadical cyclization reactions are organic chemical transformations that yield cyclic products via radical intermediates. They usually proceed in three basic steps: selective radical generation, radical cyclization, and conversion of the cyclized radical to product.-Introduction:Radical cyclization...
Example of ring-opening reactions:
- A general type of polymerization reaction: Ring-opening polymerizationRing-opening polymerizationIn polymer chemistry, ring-opening polymerization is a form of chain-growth polymerization, in which the terminal end of a polymer acts as a reactive center, where further cyclic monomers join to form a larger polymer chain through ionic propagation...
- Ring opening metathesis polymerisationRing opening metathesis polymerisationRing-opening metathesis polymerization is a type of olefin metathesis chain-growth polymerization that produces industrially important products. The driving force of the reaction is relief of ring strain in cyclic olefins and a wide variety of catalysts have been discovered...
See also
- open-chain compound
- Ring expansion and ring contraction
- MacrocycleMacrocycleA macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
- Effective molarityEffective molarityIn chemistry, the Effective Molarity is defined as the ratio between the first-order rate constant of an intramolecular reaction and the second-order rate constant of the corresponding intermolecular reaction or the ratio between the equilibrium constant of an intramolecular reaction and the...

