Dakin-West reaction
Encyclopedia
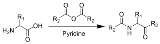
The Dakin–West reaction is a chemical reaction
that transforms an amino-acid into a keto
-amide
using an acid anhydride and a base, typically pyridine
. It is named for Henry Drysdale Dakin
(1880–1952) and Randolph West (1890–1949). Of special note, the keto-amide product is always racemic
.
 With pyridine as a base and solvent
With pyridine as a base and solvent
, refluxing conditions are required. However, with the addition of 4-dimethylaminopyridine
(DMAP) as a catalyst, the reaction can take place at room temperature.
With some acids, this reaction can take place even in the absence of an α-amino group.
This reaction should not be confused with the Dakin reaction
.
-carbon bond. Subsequent ring-opening of 6 and decarboxylation
give the final keto-amide product.

solution of the acid with catalytic N-methylimidazole. This reactivity is attributed in part to generation of acetylimidazolium, a powerful cationic acetylating agent, in situ
.

Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that transforms an amino-acid into a keto
Keto
Keto can refer to:* The Keto people, an ethnic group of the Siberian North.* Ceto or Keto, a sea goddess in Greek mythology.* Ketone or keto group, the functional group in the chemical compounds ketones....
-amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
using an acid anhydride and a base, typically pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
. It is named for Henry Drysdale Dakin
Henry Drysdale Dakin
Henry Drysdale Dakin FRS was an English chemist.He was born in London as the youngest of 8 children to a family of steel merchants from Leeds. As a school boy he did water analysis with the Leeds City Analyst. He studied chemistry at the University of Leeds with Julius B...
(1880–1952) and Randolph West (1890–1949). Of special note, the keto-amide product is always racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
.

Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, refluxing conditions are required. However, with the addition of 4-dimethylaminopyridine
4-Dimethylaminopyridine
4-Dimethylaminopyridine is a derivative of pyridine with the chemical formula 2NC5H4N. This colourless solid is a useful nucleophilic catalyst for a variety of reactions such as esterifications with anhydrides, the Baylis-Hillman reaction, hydrosilylations, tritylation, the Steglich...
(DMAP) as a catalyst, the reaction can take place at room temperature.
With some acids, this reaction can take place even in the absence of an α-amino group.
This reaction should not be confused with the Dakin reaction
Dakin reaction
The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a benzenediol and a carboxylate[3]...
.
Reaction mechanism
The reaction mechanism involves the acylation and activation of the acid 1 to the mixed anhydride 3. The amide will serve as a nucleophile for the cyclization forming the azlactone 4. Deprotonation and acylation of the azlactone forms the key carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-carbon bond. Subsequent ring-opening of 6 and decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
give the final keto-amide product.

General ketone synthesis
Modern variations on the Dakin-West reaction permit many enolizable carboxylic acids – not merely amino acids – to be converted to their corresponding methyl ketones. For example, β-aryl carboxylic acids can be efficiently converted to β-aryl ketones by treatment of an acetic anhydrideAcetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
solution of the acid with catalytic N-methylimidazole. This reactivity is attributed in part to generation of acetylimidazolium, a powerful cationic acetylating agent, in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
.


