
Danishefsky’s diene
Encyclopedia
Danishefsky’s diene is an organosilicon compound and a diene
with the formal name trans-1-methoxy-3-trimethylsilyloxy-1,3-butadiene named after Samuel J. Danishefsky
. Because the diene is very electron-rich it is a very reactive reagent
in Diels-Alder reaction
s. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride
.
The OMe group promotes highly regioselective additions.
It was first synthesized by the reaction of trimethylsilyl chloride
with 4-methoxy-3-buten-2-one and zinc chloride
:
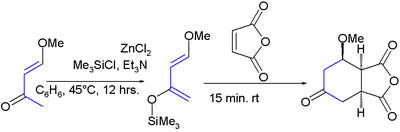 The diene has two features of interest: the substituents promote regiospecific addition to unsymmetrical dienophiles and the resulting adduct is amenable to further functional group manipulations after the addition reaction. High regioselectivity
The diene has two features of interest: the substituents promote regiospecific addition to unsymmetrical dienophiles and the resulting adduct is amenable to further functional group manipulations after the addition reaction. High regioselectivity
is obtained with unsymmetrical alkenes with a preference for an 1,2-relation of the ether group with the electron-deficient alkene-carbon. All this is exemplified in this Aza Diels-Alder reaction
:
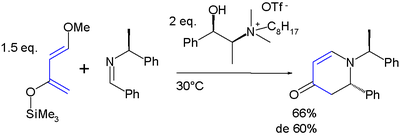 In the cycloaddition product, the silyl ether
In the cycloaddition product, the silyl ether
is a synthon
for a carbonyl
group via the enol
. The methoxy
group is susceptible to an elimination reaction
enabling the formation of a new alkene
group.
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
with the formal name trans-1-methoxy-3-trimethylsilyloxy-1,3-butadiene named after Samuel J. Danishefsky
Samuel J. Danishefsky
Samuel J. Danishefsky is an American chemist working as a professor at both Columbia University and the Memorial Sloan-Kettering Cancer Center in New York City.- Birth and education :...
. Because the diene is very electron-rich it is a very reactive reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
in Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride
Maleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
.
The OMe group promotes highly regioselective additions.
It was first synthesized by the reaction of trimethylsilyl chloride
Trimethylsilyl chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is a silyl halide, with a variety of different uses in chemistry. It has the formula 3SiCl, and under standard conditions it is a colourless liquid, which is stable in the absence of water...
with 4-methoxy-3-buten-2-one and zinc chloride
Zinc chloride
Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
:

Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
is obtained with unsymmetrical alkenes with a preference for an 1,2-relation of the ether group with the electron-deficient alkene-carbon. All this is exemplified in this Aza Diels-Alder reaction
Aza Diels-Alder reaction
The Aza Diels-Alder reaction converts imines and dienes to tetrahydropyridines. This organic reaction is a modification of the Diels-Alder reaction. The nitrogen atom can be part of the diene or the dienophile....
:

Silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis...
is a synthon
Synthon
A synthon is a concept in retrosynthetic analysis. It is defined as a structural unit within a molecule which is related to a possible synthetic operation. The term was coined by E.J. Corey...
for a carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group via the enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
. The methoxy
Methoxy
In chemistry , methoxy refers to the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula O–CH3.The word is used in organic nomenclature usually to describe an ether...
group is susceptible to an elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
enabling the formation of a new alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
group.

