
Desorption
Encyclopedia
Desorption is a phenomenon
whereby a substance is released from or through a surface. The process
is the opposite of sorption
(that is, either adsorption
and absorption). This occurs in a system being in the state of sorption equilibrium between bulk phase (fluid, i.e. gas or liquid solution) and an adsorbing surface (solid or boundary separating two fluids). When the concentration (or pressure) of substance in the bulk phase is lowered, some of the sorbed substance changes to the bulk state.
In chemistry, especially chromatography
, desorption is the ability for a chemical to move with the mobile phase. The more a chemical desorbs, the less likely it will adsorb, thus instead of sticking to the stationary phase, the chemical moves up with the solvent front.
In chemical separation process
es, stripping
is also referred to as desorption as one component of a liquid stream moves by mass transfer
into a vapor phase through the liquid-vapor interface.
After adsorption, the adsorbed chemical will remain on the substrate nearly indefinitely, provided the temperature remains low. However,as the temperature rises, so does the likelihood of desorption occurring. The general equation for the rate of desorption is:
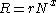 ,
,
where is the rate constant for desorption,
is the rate constant for desorption, 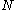 is the concentration of the adsorbed material, and
is the concentration of the adsorbed material, and  is the kinetic order of desorption.
is the kinetic order of desorption.
Usually, the order of the desorption can be predicted by the number of elementary steps involved:
Atomic or simple molecular desorption will typically be a first-order process (i.e. a simple molecule on the surface of the substrate desorbs into a gaseous form).
Recombinative molecular desorption will generally be a second-order process (i.e. two hydrogen atoms on the surface desorb and form a gaseous H2 molecule).
The rate constant may be expressed in the form
may be expressed in the form

where is the "attempt frequency" (often the Greek letter
is the "attempt frequency" (often the Greek letter 
), the chance of the adsorbed molecule overcoming its potential barrier to desorption, is the activation energy
is the activation energy
of desorption,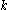 is the Boltzmann constant, and
is the Boltzmann constant, and  is the temperature.
is the temperature.
Phenomenon
A phenomenon , plural phenomena, is any observable occurrence. Phenomena are often, but not always, understood as 'appearances' or 'experiences'...
whereby a substance is released from or through a surface. The process
Process (science)
In science, a process is every sequence of changes of a real object/body which is observable using the scientific method. Therefore, all sciences analyze and model processes....
is the opposite of sorption
Sorption
Sorption refers to the action of absorption* Absorption is the incorporation of a substance in one state into another of a different state ....
(that is, either adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
and absorption). This occurs in a system being in the state of sorption equilibrium between bulk phase (fluid, i.e. gas or liquid solution) and an adsorbing surface (solid or boundary separating two fluids). When the concentration (or pressure) of substance in the bulk phase is lowered, some of the sorbed substance changes to the bulk state.
In chemistry, especially chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
, desorption is the ability for a chemical to move with the mobile phase. The more a chemical desorbs, the less likely it will adsorb, thus instead of sticking to the stationary phase, the chemical moves up with the solvent front.
In chemical separation process
Separation process
In chemistry and chemical engineering, a separation process, or simply a separation, is any mass transfer process used to convert a mixture of substances into two or more distinct product mixtures, at least one of which is enriched in one or more of the mixture's constituents. In some cases, a...
es, stripping
Stripping (chemistry)
Stripping is a physical separation process where one or more components are removed from a liquid stream by a vapor stream. In industrial applications the liquid and vapor streams can have co-current or countercurrent flows. Stripping is usually carried out in either a packed or trayed column.-...
is also referred to as desorption as one component of a liquid stream moves by mass transfer
Mass transfer
Mass transfer is the net movement of mass from one location, usually meaning a stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, adsorption, drying, precipitation, membrane filtration, and distillation. Mass transfer is used...
into a vapor phase through the liquid-vapor interface.
After adsorption, the adsorbed chemical will remain on the substrate nearly indefinitely, provided the temperature remains low. However,as the temperature rises, so does the likelihood of desorption occurring. The general equation for the rate of desorption is:
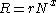 ,
,where
 is the rate constant for desorption,
is the rate constant for desorption, 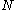 is the concentration of the adsorbed material, and
is the concentration of the adsorbed material, and  is the kinetic order of desorption.
is the kinetic order of desorption.Usually, the order of the desorption can be predicted by the number of elementary steps involved:
Atomic or simple molecular desorption will typically be a first-order process (i.e. a simple molecule on the surface of the substrate desorbs into a gaseous form).
Recombinative molecular desorption will generally be a second-order process (i.e. two hydrogen atoms on the surface desorb and form a gaseous H2 molecule).
The rate constant
 may be expressed in the form
may be expressed in the form
where
 is the "attempt frequency" (often the Greek letter
is the "attempt frequency" (often the Greek letter 
Nu (letter)
Nu , is the 13th letter of the Greek alphabet. In the system of Greek numerals it has a value of 50...
), the chance of the adsorbed molecule overcoming its potential barrier to desorption,
 is the activation energy
is the activation energyActivation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
of desorption,
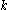 is the Boltzmann constant, and
is the Boltzmann constant, and  is the temperature.
is the temperature.See also
- AdsorptionAdsorptionAdsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
- Sorption isothermSorption isothermA sorption isotherm describes the equilibrium of the sorption of a material at a surface at constant temperature. It represents the amount of material bound at the surface as a function of the material present in the gas phase and/or in the solution...
- ChemisorptionChemisorptionChemisorption is a sub-class of adsorption, driven by a chemical reaction occurring at the exposed surface. A new chemical species is generated at the adsorbant surface...
- Gibbs isothermGibbs isothermThe Gibbs adsorption isotherm for multicomponent systems is an equation used to relate the changes in concentration of a component in contact with a surface with changes in the surface tension...
- Moisture sorption isothermMoisture sorption isothermAt equilibrium, the relationship between water content and equilibrium humidity of a material can be displayed graphically by a curve, the so called moisture sorption isotherm....
- Langmuir equationLangmuir equationThe Langmuir equation relates the coverage or adsorption of molecules on a solid surface to gas pressure or concentration of a medium above the solid surface at a fixed temperature. The equation was developed by Irving Langmuir in 1916...

