
Direct-methanol fuel cell
Encyclopedia

Proton exchange membrane fuel cell
Proton exchange membrane fuel cells, also known as polymer electrolyte membrane fuel cells , are a type of fuel cell being developed for transport applications as well as for stationary fuel cell applications and portable fuel cell applications. Their distinguishing features include lower...
in which methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
is used as the fuel. Their main advantage is the ease of transport of methanol, an energy-dense yet reasonably stable liquid at all environmental conditions.
Efficiency is presently quite low for these cells, so they are targeted especially to portable applications, where energy and power density are more important than efficiency.
A more efficient version of a direct fuel cell would play a key role in the theoretical use of methanol as a general energy transport medium, in the hypothesized (proposed) methanol economy
Methanol economy
The methanol economy is a suggested future economy in which methanol replaces fossil fuels as a means of energy storage, ground transportation fuel, and raw material for synthetic hydrocarbons and their products. It offers an alternative to the proposed hydrogen economy or ethanol economy.In the...
.
The Cell
In contrast to indirect methanol fuel cells, where methanol is reacted to hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
by steam reforming, DMFCs use a methanol solution (usually around 1M, i.e. about 3% in mass) to carry the reactant into the cell; common operating temperatures are in the range 50–120 °C, where high temperatures are usually pressurized.
DMFCs themselves are more efficient at high temperatures and pressures, but these conditions end up causing so many losses in the complete system that the advantage is lost; therefore, atmospheric-pressure configurations are currently preferred.
Because of the methanol cross-over, a phenomenon by which methanol diffuses through the membrane without reacting, methanol is fed as a weak solution: this decreases efficiency significantly, since crossed-over methanol, after reaching the air side (the cathode), immediately reacts with air; though the exact kinetics are debated, the end result is a reduction of the cell voltage.
Cross-over remains a major factor in inefficiencies, and often half of the methanol is lost to cross-over.
Other issues include the management of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
created at the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
, the sluggish dynamic behavior, and the ability to maintain the solution water.
The only waste products with these types of fuel cells are carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and water.
History
In 1990 superacidSuperacid
According to the classical definition superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a Hammett acidity function of −12. According to the modern definition, superacid is a medium, in which the chemical potential of the proton is higher than in pure...
specialist Dr. Surya Prakash
Surya Prakash
Surya Prakash or Suryaprakash is one of the Indian names.*G.Surya Prakash is a distinguished Indian Cardiologist.*P. Surya Prakash is Bishop of the Church of South India's Diocese of Karimnagar....
, and Nobel laureate Dr. George A. Olah, both of the University of Southern California
University of Southern California
The University of Southern California is a private, not-for-profit, nonsectarian, research university located in Los Angeles, California, United States. USC was founded in 1880, making it California's oldest private research university...
's Loker Hydrocarbon Research Institute
Loker Hydrocarbon Research Institute
Loker Hydrocarbon Research Institute is on the campus of the University of Southern California. Nobel Laureate George A. Olah serves as Director and G.K. Surya Prakash serves as Scientific Co-Director and holds the George A. and Judith A...
, invented a fuel cell that would directly convert methanol to electricity. USC, in a collaborative effort with Jet Propulsion Laboratory
Jet Propulsion Laboratory
Jet Propulsion Laboratory is a federally funded research and development center and NASA field center located in the San Gabriel Valley area of Los Angeles County, California, United States. The facility is headquartered in the city of Pasadena on the border of La Cañada Flintridge and Pasadena...
(JPL) proceeded to invent the direct oxidation of liquid hydrocarbons subsequently coined as DMFC, Direct Methanol Fuel Cell Technology. Others were involved in the invention, including California Institute of Technology
California Institute of Technology
The California Institute of Technology is a private research university located in Pasadena, California, United States. Caltech has six academic divisions with strong emphases on science and engineering...
(Caltech) and DTI Energy, Inc.
Some advances in DMFC have been: bringing down the fuel crossover, miniaturizing the cell for consumer and military products, ceramic plates, carbon nanotubes, porous silicon layers and oxidation reduction.
Application
Current DMFCs are limited in the power they can produce, but can still store a high energy content in a small space. This means they can produce a small amount of power over a long period of time. This makes them presently ill-suited for powering large vehicles (at least directly), but ideal for smaller vehicles such as forklifts and tuggers and consumer goods such as mobile phoneMobile phone
A mobile phone is a device which can make and receive telephone calls over a radio link whilst moving around a wide geographic area. It does so by connecting to a cellular network provided by a mobile network operator...
s, digital camera
Digital camera
A digital camera is a camera that takes video or still photographs, or both, digitally by recording images via an electronic image sensor. It is the main device used in the field of digital photography...
s or laptop
Laptop
A laptop, also called a notebook, is a personal computer for mobile use. A laptop integrates most of the typical components of a desktop computer, including a display, a keyboard, a pointing device and speakers into a single unit...
s. Military applications of DMFCs are an emerging application since they have low noise and thermal signatures and no toxic effluent. These applications include power for soldier-carried tactical equipment, battery chargers, and autonomous power for test and training instrumentation. Units are currently available with power outputs between 25 watts and 5 kilowatts with durations up to 100 hours between refuelings.
Methanol
Methanol is a liquid from -97.0 °C to 64.7 °C at atmospheric pressure.The energy density
Energy density
Energy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
of methanol is an order of magnitude greater than even highly compressed hydrogen
Compressed hydrogen
Compressed hydrogen is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar and 700 bar is used for mobile hydrogen storage in hydrogen vehicles...
, and 15 times higher than Lithium-ion batteries.
Methanol is toxic
Toxicity
Toxicity is the degree to which a substance can damage a living or non-living organisms. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell or an organ , such as the liver...
and flammable
Flammability
Flammability is defined as how easily something will burn or ignite, causing fire or combustion. The degree of difficulty required to cause the combustion of a substance is quantified through fire testing. Internationally, a variety of test protocols exist to quantify flammability...
.
However, the International Civil Aviation Organization's (ICAO) Dangerous Goods Panel (DGP) voted in November 2005 to allow passengers to carry and use micro fuel cells and methanol fuel cartridges when aboard airplanes to power laptop computers and other consumer electronic devices.
On September 24, 2007, the US Department of Transportation issued a proposal to allow airline passengers to carry fuel cell cartridges on board.
The Department of Transportation issued a final ruling on April 30, 2008, permitting passengers and crew to carry an approved fuel cell with an installed methanol cartridge and up to two additional spare cartridges.
It is worth noting that 200 ml maximum methanol cartridge volume allowed in the final ruling is double the 100 ml limit on liquids allowed by the Transportation Security Administration in carry-on bags.
Reaction
The DMFC relies upon the oxidationRedox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
on a catalyst layer to form carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
. Water is consumed at the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
and is produced at the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
. Proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s (H+) are transported across the proton exchange membrane - often made from Nafion
Nafion
Nafion is a sulfonated tetrafluoroethylene based fluoropolymer-copolymer discovered in the late 1960s by Walther Grot of DuPont. It is the first of a class of synthetic polymers with ionic properties which are called ionomers...
- to the cathode where they react with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
to produce water. Electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s are transported through an external circuit from anode to cathode, providing power to connected devices.
The half-reaction
Half-reaction
A half reaction is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction.-Example:...
s are:
!Equation
|-
!Anode
|

oxidation
|-
!Cathode
|

reduction
|-
!Overall reaction
|

redox reaction
|-
|}>
Methanol and water are adsorbed on a catalyst usually made of platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
and ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
particles, and lose protons until carbon dioxide is formed. As water is consumed at the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
in the reaction, pure methanol cannot be used without provision of water via either passive transport such as back diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
(osmosis
Osmosis
Osmosis is the movement of solvent molecules through a selectively permeable membrane into a region of higher solute concentration, aiming to equalize the solute concentrations on the two sides...
), or active transport
Active transport
Active transport is the movement of a substance against its concentration gradient . In all cells, this is usually concerned with accumulating high concentrations of molecules that the cell needs, such as ions, glucose, and amino acids. If the process uses chemical energy, such as from adenosine...
such as pumping. The need for water limits the energy density of the fuel.
Currently, platinum is used as a catalyst for both half-reactions. This contributes to the loss of cell voltage potential, as any methanol that is present in the cathode chamber will oxidize. If another catalyst could be found for the reduction of oxygen, the problem of methanol crossover would likely be significantly lessened. Furthermore, platinum is very expensive and contributes to the high cost per kilowatt of these cells.
During the methanol oxidation reaction carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
(CO) is formed, which strongly adsorbs onto the platinum catalyst, reducing the surface area and thus the performance of the cell. The addition of another components, such as ruthenium or gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
, to the catalyst tends to ameliorate this problem because, according to the most well-established theory in the field, these catalysts oxidize water to yield OH radicals: H2O → OH• + H+ + e-. The OH species from the oxidized water molecule oxidizes CO to produce CO2 which can then be released as a gas: CO + OH• → CO2 + H+ + e-.
Using these OH groups in the half reactions, they are also expressed as:
!Equation
|-
!Anode
|

oxidation
|-
!Cathode
|
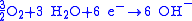
reduction
|-
!Overall reaction
|
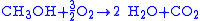
redox reaction
|-
|}>
Cross-over Current
Methanol on the anodic side is usually in a weak solution (from 1M to 3M), because methanol in high concentrations has the tendency to diffuse through the membrane to the cathode, where its concentration is about zero because it is rapidly consumed by oxygen. Low concentrations help in reducing the cross-over, but also limit the maximum attainable current.The practical realisation is usually that a solution loop enters the anode, exits, is refilled with methanol, and returns to the anode again.
Water Drag
The water in the anodic loop is lost because of the anodic reaction, but mostly because of the associated water drag: every proton formed at the anode drags a number of water molecules to the cathode. Depending on temperature and membrane type, this number can be between 2 and 6.Ancillary Units
A direct methanol fuel cell is usually part of a larger system including all the ancillary units that permit its operation. Compared to most other types of fuel cells, the ancillary system of DMFCs is relatively complex. The main reasons for its complexity are:- providing water along with methanol would make the fuel supply more cumbersome, so water has to be recycled in a loop;
- CO2 has to be removed from the solution flow exiting the fuel cell;
- water in the anodic loop is slowly consumed by reaction and drag; it is necessary to recover water from the cathodic side to maintain steady operation.
See also
- Fuel CellFuel cellA fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
- Alkali anion exchange membraneAlkali anion exchange membraneAn alkali anion exchange membrane is a semipermeable membrane generally made from ionomers and designed to conduct anions while being impermeable to gases such as oxygen or hydrogen...
- Dynamic hydrogen electrodeDynamic hydrogen electrodeA dynamic hydrogen electrode is a reference electrode, more specific a subtype of the standard hydrogen electrodes for electrochemical processes by simulating a reversible hydrogen electrode with an approximately 20 to 40 mV more negative potential....
- Glossary of fuel cell termsGlossary of fuel cell termsThe Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few. –...
- Portable fuel cell applicationsPortable fuel cell applicationsFuel cell applications are stationary fuel cell applications and portable fuel cell plications...
- Liquid fuelsLiquid fuelsLiquid fuels are those combustible or energy-generating molecules that can be harnessed to create mechanical energy, usually producing kinetic energy; they also must take the shape of their container...
- Methanol (data page)Methanol (data page)- Material Safety Data Sheet : The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet for this chemical from a reliable source such as , and follow its directions...
- Methanol economyMethanol economyThe methanol economy is a suggested future economy in which methanol replaces fossil fuels as a means of energy storage, ground transportation fuel, and raw material for synthetic hydrocarbons and their products. It offers an alternative to the proposed hydrogen economy or ethanol economy.In the...

