
Fission product
Encyclopedia
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions
. Typically, a large nucleus like that of uranium
fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat (kinetic energy
of the nuclei), gamma ray
s and neutrino
s. The two smaller nuclei are the "fission products". See Fission products (by element)
.
Occasionally a fission creates three charged products (see ternary fission
). The third product can be a smaller nucleus, most often helium
(90%) or tritium
(7%).
of the two atoms produced by the fission of one atom
is always less than the atomic weight
of the original atom. This is because some of the mass is lost as free neutron
s and large amounts of energy
.
Since the nuclei that can readily undergo fission are particularly neutron-rich (e.g. 61% of the nucleon
s in uranium-235
are neutrons), the initial fission products are almost always more neutron-rich than stable nuclei of the same mass as the fission product (e.g. stable ruthenium
-100 is 56% neutrons; stable xenon
-134 is 60%). The initial fission products therefore may be unstable and typically undergo beta decay
towards stable nuclei, converting a neutron to a proton
with each beta emission. (Fission products do not emit alpha particles.)
A few neutron-rich and short-lived initial fission products first decay by emitting a neutron. This is the source of delayed neutron
s which play an important role in control of a nuclear reactor
.
The first beta decays are rapid and may release high energy beta particle
s or gamma radiation. However, as the fission products approach stable nuclear conditions, the last one or two decays may have a long half-life
and release less energy. There are a few exceptions with relatively long half-lives and high decay energy, such as:
s with half-lives of 211,100 years (Technetium-99
) and more. Therefore the total radioactivity of fission products decreases rapidly for the first several hundred years before stabilizing at a low level that changes little for hundreds of thousands of years. This contrasts with actinides produced in the open (no nuclear reprocessing
) nuclear fuel cycle
, a number of which have half-lives in the missing range of about 100 to 200,000 years.
Proponents of nuclear fuel cycles which aim to consume all their actinides by fission, such as the Integral Fast Reactor
and molten salt reactor
, use this fact to claim that within 200 years, their wastes are no more radioactive than the original uranium ore.
Fission products emit beta radiation, while actinides primarily emit alpha radiation. Many of each also emit gamma radiation.
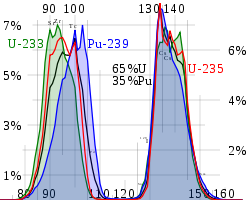 Each fission of a parent atom produces a different set of fission product atoms. However, while an individual fission is not predictable, the fission products are statistically predictable. The amount of any particular isotope produced per fission is called its yield, typically expressed as percent per parent fission; therefore, yields total to 200% not 100%.
Each fission of a parent atom produces a different set of fission product atoms. However, while an individual fission is not predictable, the fission products are statistically predictable. The amount of any particular isotope produced per fission is called its yield, typically expressed as percent per parent fission; therefore, yields total to 200% not 100%.
While fission products include every element from zinc
through the lanthanides, the majority of the fission products occur in two peaks. One peak occurs at about (expressed by atomic number) strontium
to ruthenium
while the other peak is at about tellurium to neodymium
. The yield is somewhat dependent on the parent atom and also on the energy of the initiating neutron.
In general the higher the energy of the state that undergoes nuclear fission, the more likely that the two fission products have similar mass. Hence as the neutron energy increases and/or the energy of the fissile
atom increases, the valley between the two peaks becomes more shallow.
For instance, the curve of yield against mass for Pu-239 has a more shallow valley than that observed for U-235
when the neutrons are thermal neutrons. The curves for the fission of the later actinides tend to make even more shallow valleys. In extreme cases such as 259Fm, only one peak is seen.
The adjacent figure shows a typical fission product distribution from the fission of uranium. Note that in the calculations used to make this graph, the activation of fission products was ignored and the fission was assumed to occur in a single moment rather than a length of time. In this bar chart results are shown for different cooling times — time after fission.
Because of the stability of nuclei with even numbers of protons and/or neutrons, the curve of yield against element is not a smooth curve but tends to alternate. Note that the curve against mass number is smooth.
of natural uranium, which occurs at a low rate, or as a result of neutrons from radioactive decay
or reactions with cosmic ray
particles. The microscopic tracks left by these fission products in some natural minerals (mainly apatite
and zircon
) are used in fission track dating
to provide the cooling ages of natural rocks. The technique has an effective dating
range of 0.1 Ma to >1.0 Ga depending on the mineral used and the concentration on uranium in that mineral.
About 1.5 billion years ago in a uranium ore body in Africa, a natural nuclear fission reactor
operated for a few hundred thousand years and produced approximately 5 tonnes of fission products. These fission products were important in providing proof that the natural reactor had occurred.
Fission products are produced in nuclear weapon
explosions, with the amount depending on the type of weapon.
The largest source of fission products is from nuclear reactor
s. In current nuclear power
reactors, about 3% of the uranium in the fuel is converted into fission products as a by-product of energy generation. Most of these fission products remain in the fuel unless there is fuel element failure
or a nuclear accident, or the fuel is reprocessed
.
s and activation product
s. Fission products are the largest amount of radioactivity for the first several hundred years, while actinides are dominant roughly 103 to 105 years after fuel use.
Fission occurs in the nuclear fuel, and the fission products are primarily retained within the fuel close to where they are produced. These fission products are important to the operation of the reactor because some fission products contribute delayed neutrons that are useful for reactor control while others are neutron poisons that tend to inhibit the nuclear reaction. The buildup of the fission product poisons is a key factor in determining the maximum duration a given fuel element can be kept within the reactor. The decay of short-lived fission products also provide a source of heat within the fuel that continues even after the reactor has been shut down and the fission reactions stopped. It is this decay heat
that sets the requirements for cooling of a reactor after shutdown.
If the fuel cladding around the fuel develops holes, then fission products can leak into the primary coolant
. Depending on the fission product chemistry, it may settle within the reactor core or travel through the coolant system. Coolant systems include chemistry control systems that tend to remove such fission products. In a well-designed power reactor running under normal conditions, the radioactivity of the coolant is very low.
It is known that the isotope responsible for the majority of the gamma exposure in fuel reprocessing
plants (and the Chernobyl site in 2005) is Cs-137. 129I
is one of the major radioactive elements released from reprocessing plants. In nuclear reactors both 137Cs and 90Sr
are found in locations remote from the fuel
. This is because these isotopes are formed by the beta decay
of noble gas
es (xenon-137 {halflife of 3.8 minutes} and krypton-90 {halflife 32 seconds}) which enable these isotopes to be deposited in locations remote from the fuel (e.g. on control rod
s).
s immediately) and the release of these neutrons, the latter are termed "delayed neutron
s". These delayed neutrons are important to nuclear reactor control.
Some of the fission products, such as xenon-135
and samarium-149, have a high neutron absorption capacity. Since a nuclear reactor depends on a balance in the neutron production and absorption rates, those fission products that remove neutrons from the reaction will tend to shut the reactor down or "poison" the reactor. Nuclear fuels and reactors are designed to address this phenomenon through such features as burnable poisons and control rods. Build-up of xenon-135 during shutdown or low-power operation may poison the reactor enough to impede restart
or to interfere with normal control of the reaction during restart or restoration of full power, possibly causing or contributing to an accident scenario.
s use fission as either the partial or the main energy source. Depending on the weapon design and where it is exploded, the relative importance of the fission product radioactivity will vary compared to the activation product radioactivity in the total fallout radioactivity.
The immediate fission products from nuclear weapon fission are essentially the same as those from any other fission source, depending slightly on the particular nuclide that is fissioning. However, the very short time scale for the reaction makes a difference in the particular mix of isotopes produced from an atomic bomb.
For example, the 134Cs/137Cs ratio provides an easy method of distinguishing between fallout from a bomb and the fission products from a power reactor. Almost no Cs-134 is formed by nuclear fission (because xenon
-134 is stable). The 134Cs is formed by the neutron activation
of the stable 133Cs which is formed by the decay of isotopes in the isobar
(A = 133). so in a momentary criticality by the time that the neutron
flux becomes zero too little time will have passed for any 133Cs to be present. While in a power reactor plenty of time exists for the decay of the isotopes in the isobar
to form 133Cs, the 133Cs thus formed can then be activated to form 134Cs only if the time between the start and the end of the criticality is long.
According to Jiri Hala's textbook, the radioactivity in the fission product mixture in an atom bomb is mostly caused by short-lived isotopes such as I-131
and Ba
-140. After about four months Ce
-141, Zr
-95/Nb
-95, and Sr
-89 represent the largest share of radioactive material. After two to three years, Ce
-144/Pr
-144, Ru
-106/Rh
-106, and Promethium-147 are the bulk of the radioactivity. After a few years, the radiation is dominated by Strontium-90
and Caesium-137
, whereas in the period between 10,000 and a million years it is Technetium
-99 that dominates.
99TcO4- ion can react with steel surfaces to form a corrosion resistant layer. In this way these metaloxo anions act as anodic
corrosion inhibitor
s - it renders the steel surface passive. The formation of 99TcO2 on steel
surfaces is one effect which will retard the release of 99Tc from nuclear waste drums and nuclear equipment which has become lost prior to decontamination
(e.g. nuclear submarine
reactors which have been lost at sea).
In a similar way the release of radio-iodine in a serious power reactor accident could be retarded by adsorption
on metal surfaces within the nuclear plant. A lot of other work on the iodine chemistry which would occur during a bad accident has been done.http://www.nea.fr/html/nsd/docs/2000/csni-r2000-12.pdf
 For fission of uranium-235
For fission of uranium-235
, the predominant radioactive fission products include isotopes of iodine
, caesium
, strontium
, xenon
and barium
. The threat becomes smaller with the passage of time. Locations where radiation fields once posed immediate mortal threats, such as much of the Chernobyl Nuclear Power Plant
on day one of the accident
and the ground zero
sites of U.S. atomic bombings in Japan
(6 hours after detonation) are now relatively safe because the radioactivity has decayed to a low level.
Many of the fission products decay through very short-lived isotopes to form stable isotope
s, but a considerable number of the radioisotopes have half-lives
longer than a day.
The radioactivity in the fission product mixture is mostly caused by short lived isotopes such as Iodine-131
and 140Ba, after about four months 141Ce, 95Zr/95Nb and 89Sr take the largest share, while after about two or three years the largest share is taken by 144Ce/144Pr, 106Ru/106Rh and 147Pm. Later 90Sr and 137Cs are the main radioisotopes, being succeeded by 99Tc. In the case of a release of radioactivity from a power reactor or used fuel, only some elements are released; as a result, the isotopic signature of the radioactivity is very different from an open air nuclear detonation, where all the fission products are dispersed.
is the most effective protective measure. However, if evacuation is impossible or even uncertain, then local fallout shelters and other measures provide the best protection.
 At least three isotopes of iodine
At least three isotopes of iodine
are important. 129I
, 131I
(radioiodine) and 132I. Open air nuclear testing and the Chernobyl disaster
both released iodine-131.
The short-lived isotopes of iodine are particularly harmful because the thyroid
collects and concentrates iodide
— radioactive as well as stable. Absorption of radioiodine can lead to acute, chronic, and delayed effects. Acute effects from high doses include thyroiditis
, while chronic and delayed effects include hypothyroidism
, thyroid nodule
s, and thyroid cancer
. It has been shown that the active iodine released from Chernobyl
and Mayak
has resulted in an increase in the incidence of thyroid cancer in the former Soviet Union
.
One measure which protects against the risk from radio-iodine is taking a dose of potassium iodide
before exposure to radioiodine. The non-radioactive iodide 'saturates' the thyroid, causing less of the radioiodine to be stored in the body.
Administering potassium iodide reduces the effects of radio-iodine by 99% and is a prudent, inexpensive supplement to fallout shelter
s. A low-cost alternative to commercially available iodine pills is a saturated solution of potassium iodide. Long term storage of KI is normally in the form of reagent grade crystals.
The Administration of known goitrogen
substances can also be used as a prophylaxis in reducing the bio-uptake of Iodine(whether it be non-radioactive Iodine-127 or radioactive Iodine-131, as the body cannot discern between the different Iodine isotopes). Perchlorate
ions, a common water contaminant in the USA due to the aerospace industry, has been shown to reduce Iodide uptake. Perchlorate is a competitive inhibitor of the process by which iodide, is actively deposited into thyroid follicular cells. A study involving healthy adult volunteers determined that at levels above 0.007 milligrams per kilogram per day (mg/(kg·d)), perchlorate begins to temporarily inhibit the thyroid gland’s ability to absorb iodine from the bloodstream ("iodide uptake inhibition", thus perchlorate is a known goitrogen).
The purposeful addition of ~ 250 ppb
of perchlorate ions to a regions water supply, for approximately three months, immediately after a radioiodine release, could thus be beneficial to the population in preventing radioiodine bioaccumulation
, independent of the availability of Iodate
or Iodide
drugs. In the event of a radioiodine release the ingestion of Potassium iodide or iodate, if available, would rightly take precedence and would be the first line of defense in protecting the population from a radioiodine release. However in the event of a radioiodine release too massive and widespread to be mediation by the limited stock of Iodide & Iodate prophylaxis drugs, then the addition of perchlorate ions to the water supply would serve as a cheap, efficacious, second line of defense against radioiodine bioaccumulation.
The ingestion of Goitrogen
drugs is also not without its dangers, such as Hypothyroidism
.
In both cases however, despite the risks, the prophylaxis benefits of intervention with Iodide, Iodate and Perchlorate outweight the serious cancer risk from radioiodine bioaccumulation
.
which were dispersed over a wide area. 137Cs is an isotope which is of long term concern as it remains in the top layers of soil. Plants with shallow root systems tend to absorb it for many years. Hence grass and mushrooms can carry a considerable amount of 137Cs which can be transferred to humans through the food chain
.
One of the best countermeasures in dairy farming
against 137Cs is to mix up the soil by deeply ploughing the soil. This has the effect of putting the 137Cs out of reach of the shallow roots of the grass, hence the level of radioactivity in the grass will be lowered. Also the removal of top few centimeters of soil and its burial in a shallow trench will reduce the dose to humans and animals as the gamma photon
s from 137Cs will be attenuated by their passage through the soil. The deeper and more remote the trench is, the better the degree of protection.
Fertilizer
s containing potassium
can be used to dilute caesium and limit its uptake by plants.
In livestock
farming another countermeasure against 137Cs is to feed to animals prussian blue
. This compound acts as a ion-exchanger. The cyanide
is so tightly bonded to the iron that it is safe for a human to consume several grams of prussian blue per day. The prussian blue reduces the biological half-life
(different from the nuclear half-life
) of the caesium. The physical or nuclear half-life of 137Cs is about 30 years. Caesium in humans normally has a biological half-life of between one and four months. An added advantage of the prussian blue is that the caesium which is stripped from the animal in the droppings is in a form which is not available to plants. Hence it prevents the caesium from being recycled. The form of prussian blue required for the treatment of humans or animals is a special grade. Attempts to use the pigment
grade used in paint
s have not been successful.
to soils which are poor in calcium
can reduce the uptake of strontium
by plants. Likewise in areas where the soil is low in potassium
, the addition of a potassium fertilizer can discourage the uptake of caesium into plants. However such treatments with either lime or potash
should not be undertaken lightly as they can alter the soil chemistry
greatly so resulting in a change in the plant ecology
of the land.
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
. Typically, a large nucleus like that of uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat (kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
of the nuclei), gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s and neutrino
Neutrino
A neutrino is an electrically neutral, weakly interacting elementary subatomic particle with a half-integer spin, chirality and a disputed but small non-zero mass. It is able to pass through ordinary matter almost unaffected...
s. The two smaller nuclei are the "fission products". See Fission products (by element)
Fission products (by element)
On this page, a discussion of each of the main elements in the fission product mixture from the nuclear fission of an actinide such as uranium or plutonium is set out by element.- Krypton 83-86 :Krypton-85 is formed by the fission process with...
.
Occasionally a fission creates three charged products (see ternary fission
Ternary fission
Ternary fission is a comparatively rare type of nuclear fission in which three charged products are produced rather than two...
). The third product can be a smaller nucleus, most often helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
(90%) or tritium
Tritium
Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons...
(7%).
Formation and decay
The sum of the atomic weightAtomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
of the two atoms produced by the fission of one atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
is always less than the atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
of the original atom. This is because some of the mass is lost as free neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s and large amounts of energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
.
Since the nuclei that can readily undergo fission are particularly neutron-rich (e.g. 61% of the nucleon
Nucleon
In physics, a nucleon is a collective name for two particles: the neutron and the proton. These are the two constituents of the atomic nucleus. Until the 1960s, the nucleons were thought to be elementary particles...
s in uranium-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
are neutrons), the initial fission products are almost always more neutron-rich than stable nuclei of the same mass as the fission product (e.g. stable ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
-100 is 56% neutrons; stable xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
-134 is 60%). The initial fission products therefore may be unstable and typically undergo beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
towards stable nuclei, converting a neutron to a proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
with each beta emission. (Fission products do not emit alpha particles.)
A few neutron-rich and short-lived initial fission products first decay by emitting a neutron. This is the source of delayed neutron
Delayed neutron
In nuclear engineering, a delayed neutron is a neutron emitted after a nuclear fission event by one of the fission products anytime from a few milliseconds to a few minutes later....
s which play an important role in control of a nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
.
The first beta decays are rapid and may release high energy beta particle
Beta particle
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay...
s or gamma radiation. However, as the fission products approach stable nuclear conditions, the last one or two decays may have a long half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
and release less energy. There are a few exceptions with relatively long half-lives and high decay energy, such as:
- Strontium-90 (high energy beta, half-life 30 years)
- Caesium-137 (high energy gamma, half-life 30 years)
- Tin-126 (even higher energy gamma, but long half-life of 230,000 years means a slow rate of radiation release, and the yieldFission product yieldNuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.Yield can be broken down by:#Individual isotope...
of this nuclide per fission is very low)
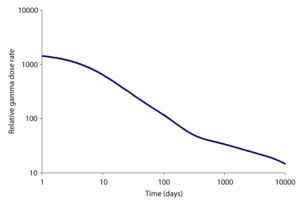
Radioactivity over time
Fission products have half-lives of 90 years (Samarium-151) or less, except for seven long-lived fission productLong-lived fission product
Long-lived fission products are radioactive materials with a long half-life produced by nuclear fission.-Evolution of radioactivity in nuclear waste:...
s with half-lives of 211,100 years (Technetium-99
Technetium-99
Technetium-99 is an isotope of technetium which decays with a half-life of 211,000 years to stable ruthenium-99, emitting soft beta rays, but no gamma rays....
) and more. Therefore the total radioactivity of fission products decreases rapidly for the first several hundred years before stabilizing at a low level that changes little for hundreds of thousands of years. This contrasts with actinides produced in the open (no nuclear reprocessing
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
) nuclear fuel cycle
Nuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in...
, a number of which have half-lives in the missing range of about 100 to 200,000 years.
Proponents of nuclear fuel cycles which aim to consume all their actinides by fission, such as the Integral Fast Reactor
Integral Fast Reactor
The Integral Fast Reactor is a design for a nuclear reactor using fast neutrons and no neutron moderator . IFR is distinguished by a nuclear fuel cycle that uses reprocessing via electrorefining at the reactor site.The U.S...
and molten salt reactor
Molten salt reactor
A molten salt reactor is a type of nuclear fission reactor in which the primary coolant, or even the fuel itself is a molten salt mixture...
, use this fact to claim that within 200 years, their wastes are no more radioactive than the original uranium ore.
Fission products emit beta radiation, while actinides primarily emit alpha radiation. Many of each also emit gamma radiation.
Yield

While fission products include every element from zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
through the lanthanides, the majority of the fission products occur in two peaks. One peak occurs at about (expressed by atomic number) strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
to ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
while the other peak is at about tellurium to neodymium
Neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite...
. The yield is somewhat dependent on the parent atom and also on the energy of the initiating neutron.
In general the higher the energy of the state that undergoes nuclear fission, the more likely that the two fission products have similar mass. Hence as the neutron energy increases and/or the energy of the fissile
Fissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
atom increases, the valley between the two peaks becomes more shallow.
For instance, the curve of yield against mass for Pu-239 has a more shallow valley than that observed for U-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
when the neutrons are thermal neutrons. The curves for the fission of the later actinides tend to make even more shallow valleys. In extreme cases such as 259Fm, only one peak is seen.
The adjacent figure shows a typical fission product distribution from the fission of uranium. Note that in the calculations used to make this graph, the activation of fission products was ignored and the fission was assumed to occur in a single moment rather than a length of time. In this bar chart results are shown for different cooling times — time after fission.
Because of the stability of nuclei with even numbers of protons and/or neutrons, the curve of yield against element is not a smooth curve but tends to alternate. Note that the curve against mass number is smooth.
Production
Small amounts of fission products are naturally formed as the result of either spontaneous fissionSpontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
of natural uranium, which occurs at a low rate, or as a result of neutrons from radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
or reactions with cosmic ray
Cosmic ray
Cosmic rays are energetic charged subatomic particles, originating from outer space. They may produce secondary particles that penetrate the Earth's atmosphere and surface. The term ray is historical as cosmic rays were thought to be electromagnetic radiation...
particles. The microscopic tracks left by these fission products in some natural minerals (mainly apatite
Apatite
Apatite is a group of phosphate minerals, usually referring to hydroxylapatite, fluorapatite, chlorapatite and bromapatite, named for high concentrations of OH−, F−, Cl− or Br− ions, respectively, in the crystal...
and zircon
Zircon
Zircon is a mineral belonging to the group of nesosilicates. Its chemical name is zirconium silicate and its corresponding chemical formula is ZrSiO4. A common empirical formula showing some of the range of substitution in zircon is 1–x4x–y...
) are used in fission track dating
Fission track dating
Fission track dating is a radiometric dating technique based on analyses of the damage trails, or tracks, left by fission fragments in certain uranium-bearing minerals and glasses...
to provide the cooling ages of natural rocks. The technique has an effective dating
Absolute dating
Absolute dating is the process of determining an approximate computed age in archaeology and geology. Some scientists prefer the terms chronometric or calendar dating, as use of the word "absolute" implies an unwarranted certainty and precision...
range of 0.1 Ma to >1.0 Ga depending on the mineral used and the concentration on uranium in that mineral.
About 1.5 billion years ago in a uranium ore body in Africa, a natural nuclear fission reactor
Natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where analysis of isotope ratios has shown that self-sustaining nuclear chain reactions have occurred. The existence of this phenomenon was discovered in 1972 at Oklo in Gabon, Africa, by French physicist Francis Perrin. The conditions under...
operated for a few hundred thousand years and produced approximately 5 tonnes of fission products. These fission products were important in providing proof that the natural reactor had occurred.
Fission products are produced in nuclear weapon
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
explosions, with the amount depending on the type of weapon.
The largest source of fission products is from nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
s. In current nuclear power
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
reactors, about 3% of the uranium in the fuel is converted into fission products as a by-product of energy generation. Most of these fission products remain in the fuel unless there is fuel element failure
Fuel element failure
A fuel element failure is a rupture in a nuclear reactor's fuel cladding that allows the nuclear fuel or fission products in the form of dissolved radioisotopes or hot particles to enter the reactor coolant or storage water....
or a nuclear accident, or the fuel is reprocessed
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
.
Power reactors
In a nuclear power reactor, the main types of radioactivity are fission products, actinideActinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s and activation product
Activation product
Activation products are materials made radioactive by neutron activation.Fission products and actinides produced by neutron absorption of nuclear fuel itself are normally referred to by those specific names, and activation product reserved for products of neutron capture by other materials, such as...
s. Fission products are the largest amount of radioactivity for the first several hundred years, while actinides are dominant roughly 103 to 105 years after fuel use.
Fission occurs in the nuclear fuel, and the fission products are primarily retained within the fuel close to where they are produced. These fission products are important to the operation of the reactor because some fission products contribute delayed neutrons that are useful for reactor control while others are neutron poisons that tend to inhibit the nuclear reaction. The buildup of the fission product poisons is a key factor in determining the maximum duration a given fuel element can be kept within the reactor. The decay of short-lived fission products also provide a source of heat within the fuel that continues even after the reactor has been shut down and the fission reactions stopped. It is this decay heat
Decay heat
Decay heat is the heat released as a result of radioactive decay. This is when the radiation interacts with materials: the energy of the alpha, beta or gamma radiation is converted into the thermal movement of atoms.-Natural occurrence:...
that sets the requirements for cooling of a reactor after shutdown.
If the fuel cladding around the fuel develops holes, then fission products can leak into the primary coolant
Coolant
A coolant is a fluid which flows through a device to prevent its overheating, transferring the heat produced by the device to other devices that use or dissipate it. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, and chemically inert, neither causing nor...
. Depending on the fission product chemistry, it may settle within the reactor core or travel through the coolant system. Coolant systems include chemistry control systems that tend to remove such fission products. In a well-designed power reactor running under normal conditions, the radioactivity of the coolant is very low.
It is known that the isotope responsible for the majority of the gamma exposure in fuel reprocessing
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
plants (and the Chernobyl site in 2005) is Cs-137. 129I
Iodine-129
Iodine-129 is long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant....
is one of the major radioactive elements released from reprocessing plants. In nuclear reactors both 137Cs and 90Sr
Strontium-90
Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard...
are found in locations remote from the fuel
Fuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
. This is because these isotopes are formed by the beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
of noble gas
Noble gas
The noble gases are a group of chemical elements with very similar properties: under standard conditions, they are all odorless, colorless, monatomic gases, with very low chemical reactivity...
es (xenon-137 {halflife of 3.8 minutes} and krypton-90 {halflife 32 seconds}) which enable these isotopes to be deposited in locations remote from the fuel (e.g. on control rod
Control rod
A control rod is a rod made of chemical elements capable of absorbing many neutrons without fissioning themselves. They are used in nuclear reactors to control the rate of fission of uranium and plutonium...
s).
Nuclear reactor poisons
Some fission products decay with the release of a neutron. Since there may be a short delay in time between the original fission event (which release its own prompt neutronPrompt neutron
In nuclear engineering, a prompt neutron is a neutron immediately emitted by a nuclear fission event, as opposed to a delayed neutron decay which can occur within the same context, emitted by one of the fission products anytime from a few milliseconds to a few minutes later.-Principle:Using U-235...
s immediately) and the release of these neutrons, the latter are termed "delayed neutron
Delayed neutron
In nuclear engineering, a delayed neutron is a neutron emitted after a nuclear fission event by one of the fission products anytime from a few milliseconds to a few minutes later....
s". These delayed neutrons are important to nuclear reactor control.
Some of the fission products, such as xenon-135
Xenon-135
Xenon-135 is an unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and Xe-135 is the most powerful known neutron-absorbing nuclear poison , with a significant effect on nuclear reactor operation...
and samarium-149, have a high neutron absorption capacity. Since a nuclear reactor depends on a balance in the neutron production and absorption rates, those fission products that remove neutrons from the reaction will tend to shut the reactor down or "poison" the reactor. Nuclear fuels and reactors are designed to address this phenomenon through such features as burnable poisons and control rods. Build-up of xenon-135 during shutdown or low-power operation may poison the reactor enough to impede restart
Iodine pit
Iodine pit, also called iodine hole and xenon pit, is a temporary disabling of a nuclear reactor due to buildup of short-lived nuclear poisons in the core of a nuclear reactor. The main isotope responsible is xenon-135, mainly produced by natural decay of iodine-135. Iodine-135 is a weak neutron...
or to interfere with normal control of the reaction during restart or restoration of full power, possibly causing or contributing to an accident scenario.
Nuclear weapons
Nuclear weaponNuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
s use fission as either the partial or the main energy source. Depending on the weapon design and where it is exploded, the relative importance of the fission product radioactivity will vary compared to the activation product radioactivity in the total fallout radioactivity.
The immediate fission products from nuclear weapon fission are essentially the same as those from any other fission source, depending slightly on the particular nuclide that is fissioning. However, the very short time scale for the reaction makes a difference in the particular mix of isotopes produced from an atomic bomb.
For example, the 134Cs/137Cs ratio provides an easy method of distinguishing between fallout from a bomb and the fission products from a power reactor. Almost no Cs-134 is formed by nuclear fission (because xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
-134 is stable). The 134Cs is formed by the neutron activation
Neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus often decays immediately by emitting particles such as neutrons, protons, or alpha...
of the stable 133Cs which is formed by the decay of isotopes in the isobar
Isobar (nuclide)
Isobars are atoms of different chemical elements that have the same number of nucleons. Correspondingly, isobars differ in atomic number but not in mass number. An example of a series of isobars would be 40S, 40Cl, 40Ar, 40K, and 40Ca...
(A = 133). so in a momentary criticality by the time that the neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
flux becomes zero too little time will have passed for any 133Cs to be present. While in a power reactor plenty of time exists for the decay of the isotopes in the isobar
Isobar (nuclide)
Isobars are atoms of different chemical elements that have the same number of nucleons. Correspondingly, isobars differ in atomic number but not in mass number. An example of a series of isobars would be 40S, 40Cl, 40Ar, 40K, and 40Ca...
to form 133Cs, the 133Cs thus formed can then be activated to form 134Cs only if the time between the start and the end of the criticality is long.
According to Jiri Hala's textbook, the radioactivity in the fission product mixture in an atom bomb is mostly caused by short-lived isotopes such as I-131
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
and Ba
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
-140. After about four months Ce
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
-141, Zr
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
-95/Nb
Niobium
Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite...
-95, and Sr
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
-89 represent the largest share of radioactive material. After two to three years, Ce
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
-144/Pr
Praseodymium
Praseodymium is a chemical element that has the symbol Pr and atomic number 59. Praseodymium is a soft, silvery, malleable and ductile metal in the lanthanide group. It is too reactive to be found in native form, and when artificially prepared, it slowly develops a green oxide coating.The element...
-144, Ru
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
-106/Rh
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
-106, and Promethium-147 are the bulk of the radioactivity. After a few years, the radiation is dominated by Strontium-90
Strontium-90
Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard...
and Caesium-137
Caesium-137
Caesium-137 is a radioactive isotope of caesium which is formed as a fission product by nuclear fission.It has a half-life of about 30.17 years, and decays by beta emission to a metastable nuclear isomer of barium-137: barium-137m . Caesium-137 is a radioactive isotope of caesium which is formed...
, whereas in the period between 10,000 and a million years it is Technetium
Technetium
Technetium is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature...
-99 that dominates.
Application
Some fission products (such as Cs-137) are used in medical and industrial radioactive sources.99TcO4- ion can react with steel surfaces to form a corrosion resistant layer. In this way these metaloxo anions act as anodic
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
corrosion inhibitor
Corrosion inhibitor
A corrosion inhibitor is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy. The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime...
s - it renders the steel surface passive. The formation of 99TcO2 on steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
surfaces is one effect which will retard the release of 99Tc from nuclear waste drums and nuclear equipment which has become lost prior to decontamination
Decontamination
Decontamination is the process of cleansing the human body to remove contamination by hazardous materials including chemicals, radioactive substances, and infectious material...
(e.g. nuclear submarine
Nuclear submarine
A nuclear submarine is a submarine powered by a nuclear reactor . The performance advantages of nuclear submarines over "conventional" submarines are considerable: nuclear propulsion, being completely independent of air, frees the submarine from the need to surface frequently, as is necessary for...
reactors which have been lost at sea).
In a similar way the release of radio-iodine in a serious power reactor accident could be retarded by adsorption
Adsorption
Adsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
on metal surfaces within the nuclear plant. A lot of other work on the iodine chemistry which would occur during a bad accident has been done.http://www.nea.fr/html/nsd/docs/2000/csni-r2000-12.pdf
Decay

Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
, the predominant radioactive fission products include isotopes of iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
, caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
, strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
, xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
and barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
. The threat becomes smaller with the passage of time. Locations where radiation fields once posed immediate mortal threats, such as much of the Chernobyl Nuclear Power Plant
Chernobyl Nuclear Power Plant
The Chernobyl Nuclear Power Plant or Chornobyl Nuclear Power Plant is a decommissioned nuclear power station near the city of Pripyat, Ukraine, northwest of the city of Chernobyl, from the Ukraine–Belarus border, and about north of Kiev. Reactor 4 was the site of the Chernobyl disaster in...
on day one of the accident
Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the Chernobyl Nuclear Power Plant in Ukraine , which was under the direct jurisdiction of the central authorities in Moscow...
and the ground zero
Ground zero
The term ground zero describes the point on the Earth's surface closest to a detonation...
sites of U.S. atomic bombings in Japan
Atomic bombings of Hiroshima and Nagasaki
During the final stages of World War II in 1945, the United States conducted two atomic bombings against the cities of Hiroshima and Nagasaki in Japan, the first on August 6, 1945, and the second on August 9, 1945. These two events are the only use of nuclear weapons in war to date.For six months...
(6 hours after detonation) are now relatively safe because the radioactivity has decayed to a low level.
Many of the fission products decay through very short-lived isotopes to form stable isotope
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
s, but a considerable number of the radioisotopes have half-lives
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
longer than a day.
The radioactivity in the fission product mixture is mostly caused by short lived isotopes such as Iodine-131
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
and 140Ba, after about four months 141Ce, 95Zr/95Nb and 89Sr take the largest share, while after about two or three years the largest share is taken by 144Ce/144Pr, 106Ru/106Rh and 147Pm. Later 90Sr and 137Cs are the main radioisotopes, being succeeded by 99Tc. In the case of a release of radioactivity from a power reactor or used fuel, only some elements are released; as a result, the isotopic signature of the radioactivity is very different from an open air nuclear detonation, where all the fission products are dispersed.
Fallout countermeasures
The purpose of radiological emergency preparedness is to protect people from the effects of radiation exposure after a nuclear accident or bomb. EvacuationEmergency evacuation
Emergency evacuation is the immediate and rapid movement of people away from the threat or actual occurrence of a hazard. Examples range from the small scale evacuation of a building due to a bomb threat or fire to the large scale evacuation of a district because of a flood, bombardment or...
is the most effective protective measure. However, if evacuation is impossible or even uncertain, then local fallout shelters and other measures provide the best protection.
Iodine

Isotopes of iodine
There are 37 known isotopes of iodine and only one, 127I, is stable. Iodine is thus a monoisotopic element.Its longest-lived radioactive isotope, 129I, has a half-life of 15.7 million years, which is far too short for it to exist as a primordial nuclide...
are important. 129I
Iodine-129
Iodine-129 is long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant....
, 131I
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
(radioiodine) and 132I. Open air nuclear testing and the Chernobyl disaster
Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the Chernobyl Nuclear Power Plant in Ukraine , which was under the direct jurisdiction of the central authorities in Moscow...
both released iodine-131.
The short-lived isotopes of iodine are particularly harmful because the thyroid
Thyroid
The thyroid gland or simply, the thyroid , in vertebrate anatomy, is one of the largest endocrine glands. The thyroid gland is found in the neck, below the thyroid cartilage...
collects and concentrates iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
— radioactive as well as stable. Absorption of radioiodine can lead to acute, chronic, and delayed effects. Acute effects from high doses include thyroiditis
Thyroiditis
Thyroiditis is the inflammation of the thyroid gland. The thyroid gland is located on the front of the neck below the laryngeal prominence, and makes hormones that control metabolism.-Classification:...
, while chronic and delayed effects include hypothyroidism
Hypothyroidism
Hypothyroidism is a condition in which the thyroid gland does not make enough thyroid hormone.Iodine deficiency is the most common cause of hypothyroidism worldwide but it can be caused by other causes such as several conditions of the thyroid gland or, less commonly, the pituitary gland or...
, thyroid nodule
Thyroid nodule
Thyroid nodules are lumps which commonly arise within an otherwise normal thyroid gland. They indicate a thyroid neoplasm, but only a small percentage of these are thyroid cancers.-Presentation:...
s, and thyroid cancer
Thyroid cancer
Thyroid neoplasm is a neoplasm or tumor of the thyroid. It can be a benign tumor such as thyroid adenoma, or it can be a malignant neoplasm , such as papillary, follicular, medullary or anaplastic thyroid cancer. Most patients are 25 to 65 years of age when first diagnosed; women are more affected...
. It has been shown that the active iodine released from Chernobyl
Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the Chernobyl Nuclear Power Plant in Ukraine , which was under the direct jurisdiction of the central authorities in Moscow...
and Mayak
Kyshtym disaster
The Kyshtym disaster was a radiation contamination incident that occurred on 29 September 1957 at Mayak, a nuclear fuel reprocessing plant in Russia...
has resulted in an increase in the incidence of thyroid cancer in the former Soviet Union
Soviet Union
The Soviet Union , officially the Union of Soviet Socialist Republics , was a constitutionally socialist state that existed in Eurasia between 1922 and 1991....
.
One measure which protects against the risk from radio-iodine is taking a dose of potassium iodide
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
before exposure to radioiodine. The non-radioactive iodide 'saturates' the thyroid, causing less of the radioiodine to be stored in the body.
Administering potassium iodide reduces the effects of radio-iodine by 99% and is a prudent, inexpensive supplement to fallout shelter
Fallout shelter
A fallout shelter is an enclosed space specially designed to protect occupants from radioactive debris or fallout resulting from a nuclear explosion. Many such shelters were constructed as civil defense measures during the Cold War....
s. A low-cost alternative to commercially available iodine pills is a saturated solution of potassium iodide. Long term storage of KI is normally in the form of reagent grade crystals.
The Administration of known goitrogen
Goitrogen
Goitrogens are substances that suppress the function of the thyroid gland by interfering with iodine uptake, which can, as a result, cause an enlargement of the thyroid, i.e., a goitre.-Goitrogenic drugs and chemicals:...
substances can also be used as a prophylaxis in reducing the bio-uptake of Iodine(whether it be non-radioactive Iodine-127 or radioactive Iodine-131, as the body cannot discern between the different Iodine isotopes). Perchlorate
Perchlorate
Perchlorates are the salts derived from perchloric acid . They occur both naturally and through manufacturing. They have been used as a medicine for more than 50 years to treat thyroid gland disorders. They are used extensively within the pyrotechnics industry, and ammonium perchlorate is also a...
ions, a common water contaminant in the USA due to the aerospace industry, has been shown to reduce Iodide uptake. Perchlorate is a competitive inhibitor of the process by which iodide, is actively deposited into thyroid follicular cells. A study involving healthy adult volunteers determined that at levels above 0.007 milligrams per kilogram per day (mg/(kg·d)), perchlorate begins to temporarily inhibit the thyroid gland’s ability to absorb iodine from the bloodstream ("iodide uptake inhibition", thus perchlorate is a known goitrogen).
The purposeful addition of ~ 250 ppb
PPB
PPB can stand for:* Party political broadcast, a type of political programming in the United Kingdom* parts-per-billion, a unit of concentration* Portland Police Bureau, a police agency for the city of Portland...
of perchlorate ions to a regions water supply, for approximately three months, immediately after a radioiodine release, could thus be beneficial to the population in preventing radioiodine bioaccumulation
Bioaccumulation
Bioaccumulation refers to the accumulation of substances, such as pesticides, or other organic chemicals in an organism. Bioaccumulation occurs when an organism absorbs a toxic substance at a rate greater than that at which the substance is lost...
, independent of the availability of Iodate
Iodate
An iodate is a conjugate base of iodic acid. In the iodate anion, iodine is bonded to three oxygen atoms and the molecular formula is IO3−. The molecular geometry of iodate is trigonal pyramidal....
or Iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
drugs. In the event of a radioiodine release the ingestion of Potassium iodide or iodate, if available, would rightly take precedence and would be the first line of defense in protecting the population from a radioiodine release. However in the event of a radioiodine release too massive and widespread to be mediation by the limited stock of Iodide & Iodate prophylaxis drugs, then the addition of perchlorate ions to the water supply would serve as a cheap, efficacious, second line of defense against radioiodine bioaccumulation.
The ingestion of Goitrogen
Goitrogen
Goitrogens are substances that suppress the function of the thyroid gland by interfering with iodine uptake, which can, as a result, cause an enlargement of the thyroid, i.e., a goitre.-Goitrogenic drugs and chemicals:...
drugs is also not without its dangers, such as Hypothyroidism
Hypothyroidism
Hypothyroidism is a condition in which the thyroid gland does not make enough thyroid hormone.Iodine deficiency is the most common cause of hypothyroidism worldwide but it can be caused by other causes such as several conditions of the thyroid gland or, less commonly, the pituitary gland or...
.
In both cases however, despite the risks, the prophylaxis benefits of intervention with Iodide, Iodate and Perchlorate outweight the serious cancer risk from radioiodine bioaccumulation
Bioaccumulation
Bioaccumulation refers to the accumulation of substances, such as pesticides, or other organic chemicals in an organism. Bioaccumulation occurs when an organism absorbs a toxic substance at a rate greater than that at which the substance is lost...
.
Caesium
The Chernobyl accident released a large amount of caesium isotopesIsotopes of caesium
Caesium has 40 known isotopes. The atomic masses of these isotopes range from 112 to 151. Only one isotope, 133Cs, is stable. The longest-lived radioisotopes are 135Cs with a half-life of 2.3 million years, 137Cs with a half-life of 30.1671 years and 134Cs with a half-life of 2.0652 years...
which were dispersed over a wide area. 137Cs is an isotope which is of long term concern as it remains in the top layers of soil. Plants with shallow root systems tend to absorb it for many years. Hence grass and mushrooms can carry a considerable amount of 137Cs which can be transferred to humans through the food chain
Food chain
A food web depicts feeding connections in an ecological community. Ecologists can broadly lump all life forms into one of two categories called trophic levels: 1) the autotrophs, and 2) the heterotrophs...
.
One of the best countermeasures in dairy farming
Dairy farming
Dairy farming is a class of agricultural, or an animal husbandry, enterprise, for long-term production of milk, usually from dairy cows but also from goats and sheep, which may be either processed on-site or transported to a dairy factory for processing and eventual retail sale.Most dairy farms...
against 137Cs is to mix up the soil by deeply ploughing the soil. This has the effect of putting the 137Cs out of reach of the shallow roots of the grass, hence the level of radioactivity in the grass will be lowered. Also the removal of top few centimeters of soil and its burial in a shallow trench will reduce the dose to humans and animals as the gamma photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
s from 137Cs will be attenuated by their passage through the soil. The deeper and more remote the trench is, the better the degree of protection.
Fertilizer
Fertilizer
Fertilizer is any organic or inorganic material of natural or synthetic origin that is added to a soil to supply one or more plant nutrients essential to the growth of plants. A recent assessment found that about 40 to 60% of crop yields are attributable to commercial fertilizer use...
s containing potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
can be used to dilute caesium and limit its uptake by plants.
In livestock
Livestock
Livestock refers to one or more domesticated animals raised in an agricultural setting to produce commodities such as food, fiber and labor. The term "livestock" as used in this article does not include poultry or farmed fish; however the inclusion of these, especially poultry, within the meaning...
farming another countermeasure against 137Cs is to feed to animals prussian blue
Prussian blue
Prussian blue is a dark blue pigment with the idealized formula Fe718. Another name for the color Prussian blue is Berlin blue or, in painting, Parisian blue. Turnbull's blue is the same substance but is made from different reagents....
. This compound acts as a ion-exchanger. The cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
is so tightly bonded to the iron that it is safe for a human to consume several grams of prussian blue per day. The prussian blue reduces the biological half-life
Biological half-life
The biological half-life or elimination half-life of a substance is the time it takes for a substance to lose half of its pharmacologic, physiologic, or radiologic activity, as per the MeSH definition...
(different from the nuclear half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
) of the caesium. The physical or nuclear half-life of 137Cs is about 30 years. Caesium in humans normally has a biological half-life of between one and four months. An added advantage of the prussian blue is that the caesium which is stripped from the animal in the droppings is in a form which is not available to plants. Hence it prevents the caesium from being recycled. The form of prussian blue required for the treatment of humans or animals is a special grade. Attempts to use the pigment
Pigment
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
grade used in paint
Paint
Paint is any liquid, liquefiable, or mastic composition which after application to a substrate in a thin layer is converted to an opaque solid film. One may also consider the digital mimicry thereof...
s have not been successful.
Strontium
The addition of limeAgricultural lime
Agricultural lime, also called aglime, agricultural limestone, garden lime or liming, is a soil additive made from pulverized limestone or chalk. The primary active component is calcium carbonate...
to soils which are poor in calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
can reduce the uptake of strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
by plants. Likewise in areas where the soil is low in potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
, the addition of a potassium fertilizer can discourage the uptake of caesium into plants. However such treatments with either lime or potash
Potash
Potash is the common name for various mined and manufactured salts that contain potassium in water-soluble form. In some rare cases, potash can be formed with traces of organic materials such as plant remains, and this was the major historical source for it before the industrial era...
should not be undertaken lightly as they can alter the soil chemistry
Soil chemistry
Soil chemistry is the study of the chemical characteristics of soil. Soil chemistry is affected by mineral composition, organic matter and environmental factors.-History:...
greatly so resulting in a change in the plant ecology
Ecology
Ecology is the scientific study of the relations that living organisms have with respect to each other and their natural environment. Variables of interest to ecologists include the composition, distribution, amount , number, and changing states of organisms within and among ecosystems...
of the land.
Health concerns
For introduction of radionuclides into organism, ingestion is the most important route. Insoluble compounds are not absorbed from the gut and cause only local irradiation before they are excreted. Soluble forms however show wide range of absorption percentages.| Isotope | Radiation | Half-life | GI Gastrointestinal tract The human gastrointestinal tract refers to the stomach and intestine, and sometimes to all the structures from the mouth to the anus. .... absorption |
Notes |
|---|---|---|---|---|
| Strontium-90 Strontium-90 Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard... /yttrium-90 Yttrium-90 Yttrium-90 is a medically significant isotope of yttrium.It emits beta rays of 2.3 MeV.Yttrium-90 is a decay product of Strontium-90 which makes up about 5% of the Nuclear daughter isotopes when Uranium is fissioned.... |
β | 28 years | 30% | |
| Caesium-137 Caesium-137 Caesium-137 is a radioactive isotope of caesium which is formed as a fission product by nuclear fission.It has a half-life of about 30.17 years, and decays by beta emission to a metastable nuclear isomer of barium-137: barium-137m . Caesium-137 is a radioactive isotope of caesium which is formed... |
β,γ | 30 years | 100% | |
| Promethium-147 | β | 2.6 years | 0.01% | |
| Cerium-144 | β,γ | 285 days | 0.01% | |
| Ruthenium-106/rhodium-106 | β,γ | 1.0 years | 0.03% | |
| Zirconium-95 | β,γ | 65 days | 0.01% | |
| Strontium-89 Strontium-89 Strontium-89 is an isotope of strontium.It is treated by the body in a similar manner to calcium, and is preferentially deposited metabolically active regions of the bone.It is an artificial radioisotope which is used in treatment of bone cancer... |
β | 51 days | 30% | |
| Ruthenium-103 | β,γ | 39.7 days | 0.03% | |
| Niobium-95 | β,γ | 35 days | 0.01% | |
| Cerium-141 | β,γ | 33 days | 0.01% | |
| Barium-140/lanthanum-140 | β,γ | 12.8 days | 5% | |
| Iodine-131 Iodine-131 Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical... |
β,γ | 8.05 days | 100% | |
| Tritium Tritium Tritium is a radioactive isotope of hydrogen. The nucleus of tritium contains one proton and two neutrons, whereas the nucleus of protium contains one proton and no neutrons... |
β | 13 years | 100% | tritiated water can absorb through skin |

