.gif)
Fission products (by element)
Encyclopedia
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
mixture from the nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
of an actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
such as uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
or plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
is set out by element.
Krypton 83-86
Krypton-85
Krypton-85
Krypton 85 is a radioisotope of krypton.It decays into rubidium-85, with a half-life of 10.756 years and a maximum decay energy of 0.687 MeV.Its most common decay is by beta particle emission with maximum energy of 687...
is formed by the fission process with
a fission yield of about 0.3%. Only 20% of the fission products of mass 85 become 85Kr itself; the rest passes through a short-lived nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
and then to stable 85Rb. If irradiated reactor fuel is reprocessed
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
, this radioactive krypton
Krypton
Krypton is a chemical element with the symbol Kr and atomic number 36. It is a member of Group 18 and Period 4 elements. A colorless, odorless, tasteless noble gas, krypton occurs in trace amounts in the atmosphere, is isolated by fractionally distilling liquified air, and is often used with other...
is released into the air. This krypton release can be detected and used as a means of detecting clandestine nuclear reprocessing. Strictly speaking, the stage which is detected is the dissolution of used nuclear fuel in nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
, as it is at this stage that the krypton and other fission gases like the more abundant xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
are released.
Increase of fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
gases above a certain limit can lead to fuel pin swelling and even puncture, so that fission gas measurement after discharging the fuel from the reactor is most important to make burn-up calculations, to study the nature of fuel inside the reactor, behaviour with pin materials, for effective utilisation of fuel and also reactor safety.
Strontium 88-90
The strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
radioisotopes are very important as strontium is a calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
mimic which is incorporated in bone growth and therefore has a great ability to harm humans. On the other hand, this also allows 89Sr to be used in the open source radiotherapy of bone tumor
Bone tumor
A bone tumor refers to a neoplastic growth of tissue in bone. Abnormal growths found in the bone can be either benign or malignant .-Classification:...
s. This tends to be used in palliative care to reduce the pain due to secondary tumor
Tumor
A tumor or tumour is commonly used as a synonym for a neoplasm that appears enlarged in size. Tumor is not synonymous with cancer...
s in the bone
Bone
Bones are rigid organs that constitute part of the endoskeleton of vertebrates. They support, and protect the various organs of the body, produce red and white blood cells and store minerals. Bone tissue is a type of dense connective tissue...
s.
Strontium-90
Strontium-90
Strontium-90 is a radioactive isotope of strontium, with a half-life of 28.8 years.-Radioactivity:Natural strontium is nonradioactive and nontoxic, but 90Sr is a radioactivity hazard...
is a strong beta
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
emitter with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 28.8 years. Its fission product yield
Fission product yield
Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.Yield can be broken down by:#Individual isotope...
decreases as the mass of the fissile
Fissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
nuclide increases. A map of 90Sr contamination around Chernobyl
Chernobyl
Chernobyl or Chornobyl is an abandoned city in northern Ukraine, in Kiev Oblast, near the border with Belarus. The city had been the administrative centre of the Chernobyl Raion since 1932....
has been published by the IAEA. http://www-pub.iaea.org/MTCD/publications/PDF/Pub886_web/Chernobylmap2.html
Yttrium 89
The only stable yttrium
Yttrium
Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is...
isotope, 89Y, will be found with yield somewhat less than 1% in a fission product mixture which has been allowed to age for months or years, as the other isotopes have half-lives of 106.6 days or less.
90Sr decays into 90Y which is a beta emitter with a half-life of 2.67 days.
90Y is sometimes used for medical purposes and can be obtained either by the neutron activation
Neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus often decays immediately by emitting particles such as neutrons, protons, or alpha...
of stable 89Y or by using a device similar to a technetium cow.
Zirconium 90-96
A significant amount of zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
is formed by the fission process; some of this are short-lived radioactives (95Zr and 97Zr which decay to molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
), while almost 10% of the fission products mixture after years of decay consists of five stable or nearly stable isotopes of zirconium plus 93Zr with a halflife of 1.53 million years which is one of the 7 major long-lived fission product
Long-lived fission product
Long-lived fission products are radioactive materials with a long half-life produced by nuclear fission.-Evolution of radioactivity in nuclear waste:...
s.
In PUREX
PUREX
PUREX is an acronym standing for Plutonium - URanium EXtraction — de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel. It is based on liquid-liquid extraction ion-exchange.The PUREX process was invented by Herbert H. Anderson and...
plants the zirconium sometimes forms a third phase
Third phase
Third phase is the term for a stable emulsion which forms in a solvent extraction system when the original two phases are mixed.The third phase can be caused by a detergent or a fine solid...
which can be a disturbance in the plant. The third phase is the term in solvent extraction given to a third layer (such as foam
Foam
-Definition:A foam is a substance that is formed by trapping gas in a liquid or solid in a divided form, i.e. by forming gas regions inside liquid regions, leading to different kinds of dispersed media...
and/or emulsion) which forms from the two layers in the solvent extraction process. The zirconium forms the third phase by forming small particles which stabilise the emulsion
Emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible . Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion is used when both the dispersed and the...
which is the third phase.
Molybdenum 95, 97, 98, 100
The fission product mixture contains significant amounts of molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
.
Technetium 99
99Tc
Technetium-99
Technetium-99 is an isotope of technetium which decays with a half-life of 211,000 years to stable ruthenium-99, emitting soft beta rays, but no gamma rays....
is produced at a yield of about 6% per fission; see also the main fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
s page.
Ruthenium 101-106
Plenty of both stable ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
and radioactive ruthenium-103 is formed by the fission process. The ruthenium in PUREX raffinate
PUREX raffinate
The term PUREX raffinate is a better term for the mixture of metals in nitric acid which are left behind when the uranium and plutonium have been removed by the PUREX process from a nuclear fuel dissolution liquor. This mixture is often known as high level nuclear waste.Two PUREX raffinates exist...
can become oxidized to form ruthenium tetroxide
Ruthenium tetroxide
Ruthenium tetroxide is a diamagnetic tetrahedral ruthenium compound. As expected for a charge-neutral symmetrical oxide, it is quite volatile. The analogous OsO4 is more widely used and better known...
which forms a purple vapour to appear above the surface of the aqueous liquor. The ruthenium tetroxide is very similar to osmium tetroxide, the ruthenium compound is a stronger oxidant which enables it to form deposits by reacting with other substances. In this way the ruthenium in a reprocessing plant is very mobile and can be found in odd places. Also at Chernobyl
Chernobyl
Chernobyl or Chornobyl is an abandoned city in northern Ukraine, in Kiev Oblast, near the border with Belarus. The city had been the administrative centre of the Chernobyl Raion since 1932....
during the fire the ruthenium became volatile and behaved differently from many of the other metallic fission products. Some of the particles which were emitted by the fire were very rich in ruthenium.
In addition the ruthenium in PUREX raffinate
Raffinate
Raffinating something is a technique used in metallurgy to remove impurities from liquid material. There are many different kinds of raffination, for example you can use vacuum to extract hydrogen from metals....
forms a large number of nitrosyl complexes which makes the chemistry of the ruthenium very complex. The ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
exchange rate at ruthenium and rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
tends to be long, hence it can take a long time for a ruthenium or rhodium compound to react.
It has been suggested that the ruthenium and palladium in PUREX raffinate should be used as a source of the metals http://www.nea.fr/html/pt/docs/iem/jeju02/session2/SessionII-14.pdfhttp://www.kiae.ru/eng/inf/tex/t52.html.
Rhodium 103
While less rhodium than ruthenium and palladium is formed (around 3.6% yield), the mixture of fission products still contains a significant amount of this metal. Due to the high prices of ruthenium, rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
and palladium some work has been done on the separation of these metals to enable them to be used at a later date. Because of the possibility of the metals being contaminated by radioactive isotopes, metals are not suitable for making consumer products such as jewellery
Jewellery
Jewellery or jewelry is a form of personal adornment, such as brooches, rings, necklaces, earrings, and bracelets.With some exceptions, such as medical alert bracelets or military dog tags, jewellery normally differs from other items of personal adornment in that it has no other purpose than to...
but this source of the metals could be used for catalysts in industrial plants such as petrochemical plants.
Potential Applications of Fission Platinoids in Industry, Zdenek Kolarik, Platinum Metals Review, 2005, 49, April (2).http://www.platinummetalsreview.com/dynamic/
A dire example of people being exposed to radiation from contaminated jewellery occurred in the USA where it is thought that the gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
seeds which were used to contain radon
Radon
Radon is a chemical element with symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas, occurring naturally as the decay product of uranium or thorium. Its most stable isotope, 222Rn, has a half-life of 3.8 days...
were recycled into jewellery. The gold did contain radioactive decay products of 222Rn. Further details can be found at http://www.orau.org/ptp/collection/hpposters/goldjewelry.htm and http://www.iaea.org/Publications/Magazines/Bulletin/Bull413/article9.pdf.
Palladium 105-110
A great deal of palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
forms during the fission process, during the fuel dissolution not all of the fission palladium dissolves. Hence the dissolver fines are rich in palladium, also some palladium dissolves at first and then comes out of solution again. The dissolver fines are often removed to prevent them disturbing the solvent extraction process through the stabilisation of the third phase
Third phase
Third phase is the term for a stable emulsion which forms in a solvent extraction system when the original two phases are mixed.The third phase can be caused by a detergent or a fine solid...
.
The fission palladium can separate during the process in which the PUREX raffinate
PUREX raffinate
The term PUREX raffinate is a better term for the mixture of metals in nitric acid which are left behind when the uranium and plutonium have been removed by the PUREX process from a nuclear fuel dissolution liquor. This mixture is often known as high level nuclear waste.Two PUREX raffinates exist...
is combined with glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
and heated to form the final waste form. The palladium forms an alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
with the fission tellurium. This alloy can separate from the glass.
Cadmium 111-116
Tin 117-126
Tellurium 125, 128, 130
Tellurium-132 and its daughter 132I are important in the first few days after a criticality. It was responsible for a large fraction of the dose inflicted on workers at Chernobyl in the first week.
The isobar
Isobar (nuclide)
Isobars are atoms of different chemical elements that have the same number of nucleons. Correspondingly, isobars differ in atomic number but not in mass number. An example of a series of isobars would be 40S, 40Cl, 40Ar, 40K, and 40Ca...
forming 132Te/132I is: Tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
-132 (half-life 40 s) decaying to antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
-132 (half-life 2.8 minutes) decaying to tellurium-132 (half-life 3.2 days) decaying to iodine-132 (half-life 2.3 hours) which decays to stable xenon-132.
Iodine 129, 131
131I
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
, with a half-life of 8 days, is a hazard from nuclear fallout
Nuclear fallout
Fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and shock wave have passed. It commonly refers to the radioactive dust and ash created when a nuclear weapon explodes...
because iodine concentrates in the thyroid gland. See also Radiation effects from Fukushima Daiichi nuclear disaster#Iodine-131 and
Downwinders#Nevada.
In common with 89Sr, 131I is used for the treatment of cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
. A small dose of 131I can be used in a thyroid function test while a large dose can be used to destroy the thyroid cancer. This treatment will also normally seek out and destroy any secondary tumor
Tumor
A tumor or tumour is commonly used as a synonym for a neoplasm that appears enlarged in size. Tumor is not synonymous with cancer...
which arose from a thyroid cancer. Much of the energy from the beta
Beta particle
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay...
emission from the 131I will be absorbed in the thyroid, while the gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s are likely to be able to escape from the thyroid to irradiate other parts of the body.
Lots of 131I was released during an experiment named the 'Green run'http://archive.tri-cityherald.com/thyroid/history.html. The 'green run' was an experiment in which fuel which had only been allowed to cool for a short time after irradiation was reprocessed in a plant which had no iodine scrubber in operation.
129I
Iodine-129
Iodine-129 is long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant....
, with a half-life almost a billion times as long, is a long-lived fission product
Long-lived fission product
Long-lived fission products are radioactive materials with a long half-life produced by nuclear fission.-Evolution of radioactivity in nuclear waste:...
.
127I is stable, the only one of the isotopes of iodine
Isotopes of iodine
There are 37 known isotopes of iodine and only one, 127I, is stable. Iodine is thus a monoisotopic element.Its longest-lived radioactive isotope, 129I, has a half-life of 15.7 million years, which is far too short for it to exist as a primordial nuclide...
that is nonradioactive.
Xenon 131-136
In reactor fuel, the fission product xenon
Xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. The element name is pronounced or . A colorless, heavy, odorless noble gas, xenon occurs in the Earth's atmosphere in trace amounts...
tends to migrate to form bubble
Liquid bubble
A bubble is a globule of one substance in another, usually gas in a liquid.Due to the Marangoni effect, bubbles may remain intact when they reach the surface of the immersive substance.-Common examples:...
s in the fuel. As caesium 133, 135, and 137 are formed by the beta particle
Beta particle
Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay...
decay of the corresponding xenon isotopes, this causes the caesium to become physically separated from the bulk of the uranium oxide fuel.
Because 135Xe is a potent nuclear poison
Nuclear poison
A neutron poison is a substance with a large neutron absorption cross-section in applications, such as nuclear reactors. In such applications, absorbing neutrons is normally an undesirable effect...
with a large cross section
Neutron cross-section
In nuclear and particle physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power...
for neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
absorption, the buildup of 135Xe in the fuel inside a power reactor can lower the reactivity greatly. If a power reactor is shut down or left running at a low power level, then large amounts of 135Xe can build up through decay of 135I. When the reactor is restarted or the low power level is increased significantly, 135Xe will be quickly consumed through neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
reactions and the reactivity of the core will increase. Under some circumstances, control systems may not be able to respond quickly enough to manage an abrupt reactivity increase as the built-up 135Xe burns off. It is thought that xenon poisoning was one of the factors which led to the power surge which damaged the Chernobyl
Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the Chernobyl Nuclear Power Plant in Ukraine , which was under the direct jurisdiction of the central authorities in Moscow...
reactor core.
Caesium 133, 134, 135, 137
A lot of caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
is formed by the fission process, please see the section in the main fission product
Fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The...
page for further details of caesium as a troublesome isotope in fall-out and nuclear wastes. This element is a key element which allows the fission products from a bomb to be distinguished from power reactor fission products.
A map of caesium-137 in the area around Chernobyl
Chernobyl
Chernobyl or Chornobyl is an abandoned city in northern Ukraine, in Kiev Oblast, near the border with Belarus. The city had been the administrative centre of the Chernobyl Raion since 1932....
has been published by the IAEA.http://www-pub.iaea.org/MTCD/publications/PDF/Pub886_web/Chernobylmap1.html
Barium 138, 139
A lot of barium
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with...
is formed by the fission process, a short lived barium isotope was confused with radium by some early workers. They were bombarding uranium with neutrons in an attempt to form a new element. But instead they caused fission which generated a large amount of radioactivity in the target. Because the chemistry of barium and radium the two elements could be coseparated by for instance a precipitation with sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
anions. Because of this similarity of their chemistry the early workers thought that the very radioactive fraction which was separated into the "radium" fraction contained a new isotope of radium. Some of this early work was done by Otto Hahn
Otto Hahn
Otto Hahn FRS was a German chemist and Nobel laureate, a pioneer in the fields of radioactivity and radiochemistry. He is regarded as "the father of nuclear chemistry". Hahn was a courageous opposer of Jewish persecution by the Nazis and after World War II he became a passionate campaigner...
and Fritz Strassmann
Fritz Strassmann
Friedrich Wilhelm "Fritz" Strassmann was a German chemist who, with Otto Hahn in 1938, identified barium in the residue after bombarding uranium with neutrons, which led to the interpretation of their results as being from nuclear fission...
.
Lanthanides (lanthanum 139, cerium 140-144, neodymium 142-146, 148, 150, promethium-147, and samarium 149, 151, 152, 154)
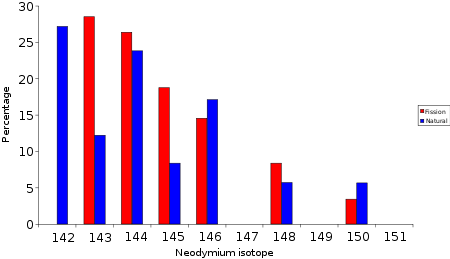
A great deal of the lighter lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s (lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
, cerium
Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight...
, neodymium
Neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is a soft silvery metal that tarnishes in air. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. It is present in significant quantities in the ore minerals monazite and bastnäsite...
, and samarium
Samarium
Samarium is a chemical element with the symbol Sm, atomic number 62 and atomic weight 150.36. It is a moderately hard silvery metal which readily oxidizes in air. Being a typical member of the lanthanide series, samarium usually assumes the oxidation state +3...
) are formed as fission products. It is interesting to note that in Africa
Africa
Africa is the world's second largest and second most populous continent, after Asia. At about 30.2 million km² including adjacent islands, it covers 6% of the Earth's total surface area and 20.4% of the total land area...
at Oklo
Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African state of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.-History:...
where the natural nuclear fission reactor
Natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where analysis of isotope ratios has shown that self-sustaining nuclear chain reactions have occurred. The existence of this phenomenon was discovered in 1972 at Oklo in Gabon, Africa, by French physicist Francis Perrin. The conditions under...
operated millions of years ago the isotopic mixture of neodymium is not the same as 'normal' neodymium, it has an isotope pattern very similar to the neodymium formed by fission.
In the aftermath of criticality accident
Criticality accident
A criticality accident, sometimes referred to as an excursion or a power excursion, is an accidental increase of nuclear chain reactions in a fissile material, such as enriched uranium or plutonium...
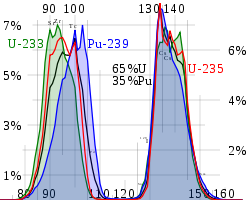
s the level of 140La is often used to determine the fission yield (in terms of the number of nuclei which underwent fission).
Samarium-149 is the second most important neutron poison in nuclear reactor physics. Samarium-151, produced at lower yields, is the third most abundant medium-lived fission product but emits only weak beta radiation. Both have high neutron absorption cross-sections, so that much of them produced in a reactor are later destroyed there by neutron absorption.

