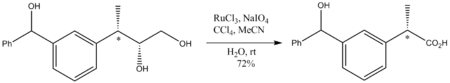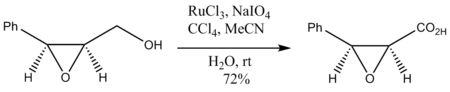
Ruthenium tetroxide
Encyclopedia
Ruthenium tetroxide is a diamagnetic
tetrahedral
ruthenium
compound. As expected for a charge-neutral symmetrical oxide
, it is quite volatile. The analogous OsO4 is more widely used and better known. One of the few solvents in which it forms stable solutions is CCl4
.
with NaIO4
.
In typical reactions featuring RuO4 as the oxidant, many forms of ruthenium usefully serve as precursors to RuO4, such as oxide hydrates or hydrated chloride.
Because RuO4 will readily decompose explosively at slightly elevated temperatures, most laboratories do not synthesize it directly, nor is it commercially available through major chemical vendors. Most laboratories instead use an anionic derivative from a salt of "TPAP" (tetrapropylammonium perruthenate), [N(C3H7)4]RuO4. TPAP is synthesized by oxidizing RuCl3 to RuO4− by NaBrO3
and countering it with the tetrapropylamine cation.
to 1-adamantanol. It is used in organic synthesis
to oxidize terminal alkynes to 1,2-diketone
s and primary alcohols to carboxylic acids. When used in this fashion, the ruthenium tetroxide is used in catalytic amounts and regenerated by the addition of sodium periodate
to ruthenium(III) chloride
and a solvent
mixture of acetonitrile
, water
and carbon tetrachloride
.
Because it is such an aggressive oxidant, reaction conditions are mild, generally room temperature. Although a strong oxidant, RuO4 oxidations do not perturb stereocenter
s that are not oxidized. Illustrative is the oxidation of the following diol to a carboxylic acid
:
Oxidation of epoxy
alcohols also occurs without degradation of the epoxide ring:
Under milder condition, oxidative reaction yields aldehyde
s instead.
RuO4 readily converts secondary alcohols into ketones. Although similar results can be achieved with other cheaper oxidants such as PCC
- or DMSO
-based oxidants, RuO4 is ideal when a very vigorous oxidant is needed but mild conditions must be maintained.
RuO4 readily cleaves double bonds to yield carbonyl
products, in a manner similar to ozonolysis
. Osmium tetroxide, a more familiar oxidant that is structurally similar to RuO4, does not cleave double bonds, instead producing vicinal
diol products.
In terms of practical details, the substrate to be oxidized is typically dissolved in solvent such as CCl4
, and acetonitrile
is added as an aiding ligand
to the catalytic cycle. Ether
can then be added to precipitate and recover the ruthenium pre-catalyst.
as a base
, RuO4 is first activated by hydroxide:
Then HRuO5− complexes with the cyclic alcohol and form a metal coordination complex (denoted C1 here):
The Ru complex is then attracted by a bromate in which the oxidation of the coordinated alcohol take place:
In which HRuO5− is reformed as the catalyst, and the cyclic alcohol is oxidized into a cyclic ketone
.
than osmium tetroxide. It has the advantage of staining even polyethylene, the disadvantage is that it is not very selective in what it stains.
Diamagnetism
Diamagnetism is the property of an object which causes it to create a magnetic field in opposition to an externally applied magnetic field, thus causing a repulsive effect. Specifically, an external magnetic field alters the orbital velocity of electrons around their nuclei, thus changing the...
tetrahedral
Tetrahedral molecular geometry
In a tetrahedral molecular geometry a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1 ≈ 109.5° when all four substituents are the same, as in CH4. This molecular geometry is common throughout the first...
ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
compound. As expected for a charge-neutral symmetrical oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
, it is quite volatile. The analogous OsO4 is more widely used and better known. One of the few solvents in which it forms stable solutions is CCl4
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
.
Preparation
RuO4 is prepared by oxidation of ruthenium(III) chlorideRuthenium(III) chloride
Ruthenium chloride is the chemical compound with the formula RuCl3. "Ruthenium chloride" more commonly refers to the hydrate RuCl3·xH2O. Both the anhydrous and hydrated species are dark brown or black solids...
with NaIO4
Sodium periodate
Sodium periodate is the sodium salt of periodic acid. It can refer to two different chemical compounds, sodium metaperiodate , which has the formula NaIO4, and sodium orthoperiodate , which has the formula Na2H3IO6...
.
- 8 Ru3+ + 5 IO4− + 12 H2O → 8 RuO4 + 5 I− + 24 H+
In typical reactions featuring RuO4 as the oxidant, many forms of ruthenium usefully serve as precursors to RuO4, such as oxide hydrates or hydrated chloride.
Because RuO4 will readily decompose explosively at slightly elevated temperatures, most laboratories do not synthesize it directly, nor is it commercially available through major chemical vendors. Most laboratories instead use an anionic derivative from a salt of "TPAP" (tetrapropylammonium perruthenate), [N(C3H7)4]RuO4. TPAP is synthesized by oxidizing RuCl3 to RuO4
Sodium bromate
Sodium bromate, the inorganic compound with the chemical formula of NaBrO3, is the sodium salt of bromic acid. It is a strong oxidant, mainly used in continuous or batch dyeing processes involving sulfur or vat dyes and as a hair-permagent, chemical agent, or gold solvent in gold mines when used...
and countering it with the tetrapropylamine cation.
Properties and uses
RuO4 oxidizes virtually any hydrocarbon. For example, it will oxidize adamantaneAdamantane
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid...
to 1-adamantanol. It is used in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
to oxidize terminal alkynes to 1,2-diketone
Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
s and primary alcohols to carboxylic acids. When used in this fashion, the ruthenium tetroxide is used in catalytic amounts and regenerated by the addition of sodium periodate
Sodium periodate
Sodium periodate is the sodium salt of periodic acid. It can refer to two different chemical compounds, sodium metaperiodate , which has the formula NaIO4, and sodium orthoperiodate , which has the formula Na2H3IO6...
to ruthenium(III) chloride
Ruthenium(III) chloride
Ruthenium chloride is the chemical compound with the formula RuCl3. "Ruthenium chloride" more commonly refers to the hydrate RuCl3·xH2O. Both the anhydrous and hydrated species are dark brown or black solids...
and a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
mixture of acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
, water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
and carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
.
Because it is such an aggressive oxidant, reaction conditions are mild, generally room temperature. Although a strong oxidant, RuO4 oxidations do not perturb stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
s that are not oxidized. Illustrative is the oxidation of the following diol to a carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
:
Oxidation of epoxy
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
alcohols also occurs without degradation of the epoxide ring:
Under milder condition, oxidative reaction yields aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s instead.
RuO4 readily converts secondary alcohols into ketones. Although similar results can be achieved with other cheaper oxidants such as PCC
Pyridinium chlorochromate
Pyridinium chlorochromate is a reddish orange solid reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. Pyridinium chlorochromate, or PCC, will not fully oxidize a primary alcohol to the carboxylic acid as does the Jones reagent. A disadvantage to using PCC is...
- or DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
-based oxidants, RuO4 is ideal when a very vigorous oxidant is needed but mild conditions must be maintained.
RuO4 readily cleaves double bonds to yield carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
products, in a manner similar to ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
. Osmium tetroxide, a more familiar oxidant that is structurally similar to RuO4, does not cleave double bonds, instead producing vicinal
Vicinal
Vicinal may refer to:* Vicinal , stands for any two functional groups bonded to two adjacent atoms.* Vicinal , a word where all letters have alphabetic neighbors....
diol products.
In terms of practical details, the substrate to be oxidized is typically dissolved in solvent such as CCl4
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
, and acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
is added as an aiding ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
to the catalytic cycle. Ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
can then be added to precipitate and recover the ruthenium pre-catalyst.
Oxidative catalyst and mechanism
Although used as a direct oxidant, due to the relatively high cost of RuO4 it is also used catalytically with an associated re-oxidant. For an oxidation of cyclic alcohols with RuO4 as a catalyst and bromateBromate
The bromate anion, BrO, is a bromine-based oxoanion. A bromate is a chemical compound that contains this ion. Examples of bromates include sodium bromate, , and potassium bromate, .Bromates are formed many different ways in municipal drinking water...
as a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
, RuO4 is first activated by hydroxide:
- RuO4 + OH− → HRuO5−
Then HRuO5− complexes with the cyclic alcohol and form a metal coordination complex (denoted C1 here):
- HRuO5− + (CH2CH2)nCHOH → C1 + OH−
The Ru complex is then attracted by a bromate in which the oxidation of the coordinated alcohol take place:
- C1 + BrO3− → (CH2CH2)nC=O + HRuO5−
In which HRuO5− is reformed as the catalyst, and the cyclic alcohol is oxidized into a cyclic ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
.
Staining
Ruthenium tetroxide can be used as an even more aggressive form of staining for the study of polymers by Transmission Electron MicroscopyTransmission electron microscopy
Transmission electron microscopy is a microscopy technique whereby a beam of electrons is transmitted through an ultra thin specimen, interacting with the specimen as it passes through...
than osmium tetroxide. It has the advantage of staining even polyethylene, the disadvantage is that it is not very selective in what it stains.