
Adamantane
Encyclopedia
Adamantane is a colorless, crystalline chemical compound
with a camphor
-like odor. With a formula C10H16, it is a cycloalkane
and also the simplest diamondoid
. Adamantane molecules consist of three cyclohexane
rings arranged in the "armchair" configuration. It is unique in that it is both rigid and virtually stress-free. Adamantane is the most stable among all the isomers with formula C10H16, which include the somewhat similar twistane
. The spatial arrangement of carbon
atoms is the same in adamantane molecule and in the diamond
crystal. This motivates the name adamantane, which is derived from the Greek adamantinos (relating to steel
or diamond
).
The discovery of adamantane in petroleum
in 1933 launched a new chemistry field studying the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials and thermally stable lubricants.
with the C10H16 formula and diamond-like structure of the molecule was suggested by H. Decker at a conference in 1924. Dekker called this molecule decaterpene and was surprised that it had not been synthesized yet.
The first attempt of laboratory synthesis was made by German chemist Hans Meerwein
in 1924 using reaction of formaldehyde
with diethyl malonate
in the presence of piperidine
. Instead of adamantane, Meerwein obtained 1,3,5,7-tetracarbomethoxybicyclo[3.3.1]nonane-2,6-dione. This compound was later named Meerwein's ester and used in the syntheses of adamantane and its derivatives. Later, another German chemist D. Bottger tried to obtain adamantane using Meerwein's ester as precursor. However, the product, tricyclo-[3.3.1.13,7] decane ring system, was again an adamantane derivative.
Other researchers attempted to synthesize adamantane using phloroglucinol
and derivatives of cyclohexanone
but also without success.
Adamantane was first synthesized by Vladimir Prelog
in 1941 from Meerwein's ester. The process was impractical as it contained five stages (simplified in the image below) and had a yield of about 0.16%. However, it was sometimes used to synthesize certain derivatives of adamantane.
Prelog's method was refined in 1956. The decarboxylation yield was increased by the addition of the Heinsdecker pathway (11%), and the Hoffman reaction (24%) that raised the total yield to 6.5%. The process was still too complex, and a more convenient method was accidentally found by Paul von Ragué Schleyer
in 1957: dicyclopentadiene
was first hydrogenated
in the presence of a catalyst (e.g. platinum dioxide
) and then transformed into adamantane using a Lewis acid (e.g. aluminium chloride
) as another catalyst. This method increased the yield to 30–40% and provided an affordable source of adamantane; it therefore stimulated characterization of adamantane and is still used in the laboratory practice. The adamantane synthesis yield was later increased to 60% and nowadays, adamantane is an affordable chemical compound with a cost of the order $1/gram.
All the above methods yield adamantane in the form of polycrystalline powder. Using this powder, single crystals can be grown from the melt, solution or vapor phase (e.g. with the Bridgman–Stockbarger technique). Melt growth result in the worst crystalline quality with a mosaic spread in the X-ray reflection of about 1°. Best crystals are obtained from the liquid phase, but the growth is inpracticably slow – several months for a 5–10 mm crystal. Growth from the vapor phase is a reasonable compromise in terms of speed and quality. Adamantane is sublimated in a quartz tube placed in a furnace, which is equipped with several heaters maintaining a certain temperature gradient (about 10 °C/cm for adamantane) along the tube. Crystallization starts at one end of the tube which is kept near the freezing point of adamantane. Slow cooling of the tube, while maintaining the temperature gradient, gradually shifts the melting zone (rate ~2 mm/hour) producing a single-crystal boule
.
where a sample of petroleum was gradually heated until leaving only solid impurities. The resulting steam enters a fractional distillation column. The temperature of the column decreases as the steam rises through the column. Therefore, hydrocarbon fractions condense along the column corresponding to their boiling point. Landa and Machacek could produce only a few milligrams of adamantane but noticed its high boiling and melting point
s. Because of the (assumed) similarity of its structure to that of diamond, the new compound was named adamantane.
Petroleum remains the only natural source of adamantane; the content varies between 0.0001 and 0.03% depending on the oil field and is too low for commercial production.
Beside adamantane, petroleum contains more than thirty of its derivatives. Their isolation from a complex mixture of hydrocarbons is possible due to their high melting point and the ability to distill with water vapor and form stable adduct
s with thiourea
.
for a hydrocarbon
. At 270 °C, its melting point is much higher than other hydrocarbons with the same molecular weight, such as camphene
(45 °C), limonene
(–74 °C), ocimene
(50 °C), terpinene
(60 °C) or twistane
(164 °C), or than a linear C10H22 hydrocarbon decane
(–28 °C). However, adamantane slowly sublimates even at room temperature. Adamantane can distill with water vapor
.
rings fused in the chair conformation
. The molecular parameters were deduced by electron diffraction
and X-ray crystallography
. The carbon-carbon bond length is 1.54 Å
and is almost identical to that of diamond, and the carbon-hydrogen distance is 1.112 Å.
At ambient conditions, adamantane crystallizes in a face-centered cubic structure (space group
Fm3m, a = 9.426 ± 0.008 Å
, four molecules in the unit cell) containing orientationally disordered adamantane molecules. This structure transforms into an ordered body-centered tetragonal phase (a = 6.641 Å
, c = 8.875 Å
) with two molecules per cell either upon cooling to 208 K or pressurizing to above 0.5 GPa.
This phase transition
is of the first order; it is accompanied by an anomaly in the heat capacity
, elastic and other properties. In particular, whereas adamantane molecules freely rotate in the cubic phase, they are frozen in the tetragonal one; the density increases stepwise from 1.08 to 1.18 g/cm3 and the entropy
changes by a significant amount of 1594 J/(mol·K).
, C11, was deduced as 7.52, 8.20 and 6.17 GPa for the <110>, <111> and <100> crystalline directions. For comparison, the corresponding values for crystalline diamond are 1161, 1174 and 1123 GPa. The arrangement of carbon atoms is the same in adamantane and diamond. However, in the adamantane solid, molecules do not form a covalent lattice as in diamond, but interact through weak Van der Waals force
s. As a result, adamantane crystals are very soft and plastic.
(NMR) spectrum of adamantane consists of two poorly resolved signals, which correspond to the inequivalent sites 1 and 2 (see picture below). Their positions are 1.873 ppm and 1.756 ppm for adamantane in CDCl3 and 1H NMR, and are 28.46 ppm and 37.85 ppm for 13C NMR. The simplicity of the NMR spectrum is a good monitor of the purity of adamantane – most derivatives have lower molecular symmetry and therefore more complex spectra.
Mass spectra
of adamantane and its derivatives are rather characteristic. The main peak at m/z = 136 corresponds to the ion. Its fragmentation results in weaker signals as m/z = 93, 80, 79, 67, 41 and 39.
The infrared absorption spectrum
of adamantane is relatively simple because of the high symmetry of the molecule. The main absorption bands and their assignment are given in the table.
and optically active. As in biphenyl
s, the center of chirality does not belong to any particular carbon atom. Such optical activity was described in adamantane in 1969 with the four different substituents being hydrogen
, bromine
and methyl and carboxyl group. The values of specific rotation
are small and are usually within 1°.
The adamantane molecule is composed of only carbon and hydrogen and has high Td symmetry. Therefore, its 16 hydrogen and 10 carbon atoms can be described by only two sites, which are labeled in the figure as 1 (4 equivalent sites) and 2 (6 equivalent sites).
The closest structural analogs of adamantane are noradamantane and homoadamantane, which respectively contain one less and one more CH2 link than the adamantane.
s are formed as intermediates.
The dication
of adamantane was obtained in solutions of superacid
s. It also has elevated stability due to the phenomenon called "three-dimensional aromaticity".
which produces adamantanone.
The carbonyl group
of adamantanone allows further reactions via the bridging site. For example, adamantanone is the starting compound for obtaining such derivatives of adamantane as 2-adamantanecarbonitrile and 2-methyl-adamantane.
. The composition and the ratio of the reaction products depend on the reaction conditions and especially presence of catalyst s.
Boiling of adamantane with bromine results in a monosubstituted adamantane, 1-bromadamantane. Multiple substitution with bromine is achieved by adding a Lewis acid
catalyst.
The rate of bromination is accelerated upon addition of Lewis acids and is unchanged by irradiation or addition of free radicals. This indicates that the reaction occurs via an ionic mechanism.
has also been reported.
as a carboxylating agent and carbon tetrachloride
as a solvent.
tert-butanol
(t-BuOH) and sulfuric acid were added to generate adamantane cation; the cation was then carboxylated by carbon monoxide
generated in situ in the interaction between the formic
and sulfuric acid
s. The fraction of carboxylated adamantane was 55-60%.1-Adamantanecarboxylic acid Organic Syntheses, Coll. Vol. 5, p. 20 (1973); Vol. 44, p. 1 (1964).
. It can also be produced by ozonation of the adamantane:
in the presence of Lewis acids, resulting in a Friedel–Crafts reaction. Aromatically substituted adamantane derivatives can be easily obtained starting from 1-hydroxyadamantane. In particular, the reaction with anisole
proceeds under normal conditions and does not require a catalyst.
Nitration of adamantane is a difficult reaction characterized by moderate yields. An important nitrogen-substituted drug amantadine
can be prepared by reacting adamantane with bromine or nitric acid
to give the bromide or nitroester at the 1- position. Reaction of either compound with acetonitrile
affords the acetamide, which is hydrolyzed to give 1-adamantylamine:
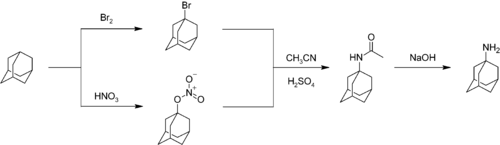
. It is used in some dry etching
masks and polymer
formulations.
In solid-state NMR spectroscopy, adamantane is a common standard for chemical shift
referencing. In dye laser
s, adamantane may be used to extend the life of the gain medium; it cannot be photoionized under atmosphere because its absorption bands lie in the vacuum-ultraviolet region of the spectrum. Photoionization energies have been determined for adamantane as well as for several bigger diamondoids.
– first (1967) as an antiviral drug
against various strains of flu and then to treat the Parkinson's disease
. Other drugs among adamantane derivatives include memantine, rimantadine
, dopamantin, tromantadine, vildagliptin and karmantadin. Polymer
s of adamantane have been patented as antiviral agents against HIV
.
. Adamantane-based polymers might find application for coatings of touchscreen
s, and there are prospects for using adamantane and its homologues in nanotechnology
. For example, the soft cage-like structure of adamantane solid allow incorporation of guest molecules, which can be released inside the human body upon breaking the matrix.
P4O6, arsenic trioxide
As4O6, phosphorus pentoxide
P4O10 = (PO)4O6, phosphorus pentasulfide
P4S10 = (PS)4S6, and hexamethylenetetramine C6N4H12 = N4(CH2)6. Particularly notorious is tetramethylenedisulfotetramine
, often shortened to "tetramine", a rodenticide banned in most countries for extreme toxicity to humans. The silicon analogue of adamantane, sila-adamantane, was synthesized in 2005.
Adamantane cages can be stacked together to produce higher diamondoids, such as diamantane (C14H20 – two fused adamantane cages), triamantane (C18H24), tetramantane (C22H28), pentamantane (C26H32), hexamantane (C26H30), etc. Their synthesis is similar to that of adamantane and like adamantane, they can also be extracted from petroleum, though at even much smaller yields.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with a camphor
Camphor
Camphor is a waxy, white or transparent solid with a strong, aromatic odor. It is a terpenoid with the chemical formula C10H16O. It is found in wood of the camphor laurel , a large evergreen tree found in Asia and also of Dryobalanops aromatica, a giant of the Bornean forests...
-like odor. With a formula C10H16, it is a cycloalkane
Cycloalkane
Cycloalkanes are types of alkanes that have one or more rings of carbon atoms in the chemical structure of their molecules. Alkanes are types of organic hydrocarbon compounds that have only single chemical bonds in their chemical structure...
and also the simplest diamondoid
Diamondoid
A diamondoid, in the context of building materials for nanotechnology components, most generally refers to structures that resemble diamond in a broad sense: namely, strong, stiff structures containing dense, 3-D networks of covalent bonds, formed chiefly from first and second row atoms with a...
. Adamantane molecules consist of three cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
rings arranged in the "armchair" configuration. It is unique in that it is both rigid and virtually stress-free. Adamantane is the most stable among all the isomers with formula C10H16, which include the somewhat similar twistane
Twistane
Twistane is an organic compound with the formula C10H16. It is a cycloalkane and an isomer of the simplest diamondoid, adamantane, and like adamantane, is very volatile...
. The spatial arrangement of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms is the same in adamantane molecule and in the diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
crystal. This motivates the name adamantane, which is derived from the Greek adamantinos (relating to steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
or diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
).
The discovery of adamantane in petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
in 1933 launched a new chemistry field studying the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials and thermally stable lubricants.
History and synthesis
The possibility of the existence of a hydrocarbonHydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
with the C10H16 formula and diamond-like structure of the molecule was suggested by H. Decker at a conference in 1924. Dekker called this molecule decaterpene and was surprised that it had not been synthesized yet.
The first attempt of laboratory synthesis was made by German chemist Hans Meerwein
Hans Meerwein
Hans Meerwein was a German chemist.His name is present in the names of several reactions and reagents, for example the Meerwein-Ponndorf-Verley reduction, the Wagner-Meerwein rearrangement...
in 1924 using reaction of formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
with diethyl malonate
Diethyl malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes...
in the presence of piperidine
Piperidine
Piperidine is an organic compound with the molecular formula 5NH. This heterocyclic amine consists of a six-membered ring containing five methylene units and one nitrogen atom...
. Instead of adamantane, Meerwein obtained 1,3,5,7-tetracarbomethoxybicyclo[3.3.1]nonane-2,6-dione. This compound was later named Meerwein's ester and used in the syntheses of adamantane and its derivatives. Later, another German chemist D. Bottger tried to obtain adamantane using Meerwein's ester as precursor. However, the product, tricyclo-[3.3.1.13,7] decane ring system, was again an adamantane derivative.
Other researchers attempted to synthesize adamantane using phloroglucinol
Phloroglucinol
Phloroglucinol is a benzenetriol. It is an organic compound that is used in the synthesis of pharmaceuticals and explosives. This molecule exists in two forms, or tautomers, 1,3,5-trihydroxybenzene, which has phenol-like, and 1,3,5-cyclohexanetrione , which has ketone-like character. These two...
and derivatives of cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
but also without success.
Adamantane was first synthesized by Vladimir Prelog
Vladimir Prelog
Vladimir Prelog FRS was a Croatian chemist and Nobel Prize winner in chemistry. Prelog lived and worked in Prague, Zagreb and Zürich during his lifetime.-Biography:...
in 1941 from Meerwein's ester. The process was impractical as it contained five stages (simplified in the image below) and had a yield of about 0.16%. However, it was sometimes used to synthesize certain derivatives of adamantane.
Prelog's method was refined in 1956. The decarboxylation yield was increased by the addition of the Heinsdecker pathway (11%), and the Hoffman reaction (24%) that raised the total yield to 6.5%. The process was still too complex, and a more convenient method was accidentally found by Paul von Ragué Schleyer
Paul von Rague Schleyer
Paul von Ragué Schleyer is an organic physical chemist of substantial significance whose research has been cited with great frequency. A 1997 survey indicated that Dr. Schleyer was, at the time, the world's third most cited chemist, with over 1100 technical papers produced...
in 1957: dicyclopentadiene
Dicyclopentadiene
Dicyclopentadiene, abbreviated DCPD, is a chemical compound with formula C10H12. At room temperature, it is a white crystalline solid with a camphor-like odor. Its energy density is 10,975 Wh/l....
was first hydrogenated
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
in the presence of a catalyst (e.g. platinum dioxide
Adams' catalyst
Adams' catalyst, also known as platinum dioxide, is usually represented as platinum oxide hydrate, PtO2-H2O. It is a catalyst for hydrogenation and hydrogenolysis in organic synthesis. This dark brown powder is commercially available...
) and then transformed into adamantane using a Lewis acid (e.g. aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
) as another catalyst. This method increased the yield to 30–40% and provided an affordable source of adamantane; it therefore stimulated characterization of adamantane and is still used in the laboratory practice. The adamantane synthesis yield was later increased to 60% and nowadays, adamantane is an affordable chemical compound with a cost of the order $1/gram.
All the above methods yield adamantane in the form of polycrystalline powder. Using this powder, single crystals can be grown from the melt, solution or vapor phase (e.g. with the Bridgman–Stockbarger technique). Melt growth result in the worst crystalline quality with a mosaic spread in the X-ray reflection of about 1°. Best crystals are obtained from the liquid phase, but the growth is inpracticably slow – several months for a 5–10 mm crystal. Growth from the vapor phase is a reasonable compromise in terms of speed and quality. Adamantane is sublimated in a quartz tube placed in a furnace, which is equipped with several heaters maintaining a certain temperature gradient (about 10 °C/cm for adamantane) along the tube. Crystallization starts at one end of the tube which is kept near the freezing point of adamantane. Slow cooling of the tube, while maintaining the temperature gradient, gradually shifts the melting zone (rate ~2 mm/hour) producing a single-crystal boule
Boule (crystal)
A boule is a single-crystal ingot produced by synthetic means. A boule of silicon is the starting material for most of the integrated circuits used today....
.
Natural occurrence
Before adamantane was synthesized, it was isolated from petroleum by Czech chemists S. Landa and V. Machacek in 1933. They used fractional distillationFractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions, such as in separating chemical compounds by their boiling point by heating them to a temperature at which several fractions of the compound will evaporate. It is a special type of distillation...
where a sample of petroleum was gradually heated until leaving only solid impurities. The resulting steam enters a fractional distillation column. The temperature of the column decreases as the steam rises through the column. Therefore, hydrocarbon fractions condense along the column corresponding to their boiling point. Landa and Machacek could produce only a few milligrams of adamantane but noticed its high boiling and melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
s. Because of the (assumed) similarity of its structure to that of diamond, the new compound was named adamantane.
Petroleum remains the only natural source of adamantane; the content varies between 0.0001 and 0.03% depending on the oil field and is too low for commercial production.
Beside adamantane, petroleum contains more than thirty of its derivatives. Their isolation from a complex mixture of hydrocarbons is possible due to their high melting point and the ability to distill with water vapor and form stable adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
s with thiourea
Thiourea
Thiourea is an organosulfur compound of with the formula SC2 . It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad...
.
Physical properties
Pure adamantane is a colorless crystalline solid with a characteristic camphor smell. It is practically insoluble in water, but readily soluble in nonpolar organic solvents. Adamantane has an unusually high melting pointMelting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
for a hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
. At 270 °C, its melting point is much higher than other hydrocarbons with the same molecular weight, such as camphene
Camphene
Camphene is bicyclic monoterpene. It is nearly insoluble in water, but very soluble in common organic solvents. It volatilizes readily at room temperature and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil,...
(45 °C), limonene
Limonene
Limonene is a colourless liquid hydrocarbon classified as a cyclic terpene. The more common D isomer possesses a strong smell of oranges. It is used in chemical synthesis as a precursor to carvone and as a renewably-based solvent in cleaning products....
(–74 °C), ocimene
Ocimene
Ocimene refers to several isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is 3,7-dimethyl-1,3,7-octatriene. ...
(50 °C), terpinene
Terpinene
The terpinenes are three isomeric hydrocarbons that are classified as terpenes. They each have the same molecular formula and carbon framework, but they differ in the position of carbon-carbon double bonds. α-Terpinene has been isolated from cardamom and marjoram oils, and from other natural...
(60 °C) or twistane
Twistane
Twistane is an organic compound with the formula C10H16. It is a cycloalkane and an isomer of the simplest diamondoid, adamantane, and like adamantane, is very volatile...
(164 °C), or than a linear C10H22 hydrocarbon decane
Decane
Decane is an alkane hydrocarbon with the chemical formula CH38CH3.75 structural isomers of decane exist, all of which are flammable liquids. Decane is one of the components of gasoline . Like other alkanes, it is nonpolar and therefore will not dissolve in polar liquids such as water...
(–28 °C). However, adamantane slowly sublimates even at room temperature. Adamantane can distill with water vapor
Steam distillation
Steam distillation is a special type of distillation for temperature sensitive materials like natural aromatic compounds....
.
Structure
Adamantane molecule consists of three condensed cyclohexaneCyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
rings fused in the chair conformation
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
. The molecular parameters were deduced by electron diffraction
Electron diffraction
Electron diffraction refers to the wave nature of electrons. However, from a technical or practical point of view, it may be regarded as a technique used to study matter by firing electrons at a sample and observing the resulting interference pattern...
and X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
. The carbon-carbon bond length is 1.54 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
and is almost identical to that of diamond, and the carbon-hydrogen distance is 1.112 Å.
At ambient conditions, adamantane crystallizes in a face-centered cubic structure (space group
Space group
In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct...
Fm3m, a = 9.426 ± 0.008 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
, four molecules in the unit cell) containing orientationally disordered adamantane molecules. This structure transforms into an ordered body-centered tetragonal phase (a = 6.641 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
, c = 8.875 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
) with two molecules per cell either upon cooling to 208 K or pressurizing to above 0.5 GPa.
This phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
is of the first order; it is accompanied by an anomaly in the heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
, elastic and other properties. In particular, whereas adamantane molecules freely rotate in the cubic phase, they are frozen in the tetragonal one; the density increases stepwise from 1.08 to 1.18 g/cm3 and the entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
changes by a significant amount of 1594 J/(mol·K).
Hardness
Elastic constants of adamantane were measured using large (centimeter sized) single crystals and the ultrasonic echo technique. The principal value of the elasticity tensorLinear elasticity
Linear elasticity is the mathematical study of how solid objects deform and become internally stressed due to prescribed loading conditions. Linear elasticity models materials as continua. Linear elasticity is a simplification of the more general nonlinear theory of elasticity and is a branch of...
, C11, was deduced as 7.52, 8.20 and 6.17 GPa for the <110>, <111> and <100> crystalline directions. For comparison, the corresponding values for crystalline diamond are 1161, 1174 and 1123 GPa. The arrangement of carbon atoms is the same in adamantane and diamond. However, in the adamantane solid, molecules do not form a covalent lattice as in diamond, but interact through weak Van der Waals force
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
s. As a result, adamantane crystals are very soft and plastic.
Spectroscopy
The nuclear magnetic resonanceNuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
(NMR) spectrum of adamantane consists of two poorly resolved signals, which correspond to the inequivalent sites 1 and 2 (see picture below). Their positions are 1.873 ppm and 1.756 ppm for adamantane in CDCl3 and 1H NMR, and are 28.46 ppm and 37.85 ppm for 13C NMR. The simplicity of the NMR spectrum is a good monitor of the purity of adamantane – most derivatives have lower molecular symmetry and therefore more complex spectra.
Mass spectra
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
of adamantane and its derivatives are rather characteristic. The main peak at m/z = 136 corresponds to the ion. Its fragmentation results in weaker signals as m/z = 93, 80, 79, 67, 41 and 39.
The infrared absorption spectrum
Infrared spectroscopy
Infrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
of adamantane is relatively simple because of the high symmetry of the molecule. The main absorption bands and their assignment are given in the table.
Optical activity
If adamantane molecules have four different substituents at every nodal carbon site then they are chiralChirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
and optically active. As in biphenyl
Biphenyl
Biphenyl is an organic compound that forms colorless crystals. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula 2...
s, the center of chirality does not belong to any particular carbon atom. Such optical activity was described in adamantane in 1969 with the four different substituents being hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
and methyl and carboxyl group. The values of specific rotation
Specific rotation
In stereochemistry, the specific rotation of a chemical compound [α] is defined as the observed angle of optical rotation α when plane-polarized light is passed through a sample with a path length of 1 decimeter and a sample concentration of 1 gram per 1 millilitre. It is the main property used to...
are small and are usually within 1°.
Nomenclature
According to the rules of systematic nomenclature, adamantane should be called tricyclo[3.3.1.13,7]decane. However, IUPAC recommends using the name "adamantane".The adamantane molecule is composed of only carbon and hydrogen and has high Td symmetry. Therefore, its 16 hydrogen and 10 carbon atoms can be described by only two sites, which are labeled in the figure as 1 (4 equivalent sites) and 2 (6 equivalent sites).
The closest structural analogs of adamantane are noradamantane and homoadamantane, which respectively contain one less and one more CH2 link than the adamantane.
Chemical properties
Usually, hydrocarbons which contain only σ-bonds are relatively inert chemically. However, adamantane and its derivatives are highly reactive. This property is particularly evident in the ionic reactions where carbocationCarbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s are formed as intermediates.
Adamantane cations
The adamantane cation can be produced by reacting 1-fluoro-adamantane with SbF5 and it has high stability compared with other tertiary carbocations.The dication
Dication
A dication is any cation, of general formula 2+, formed by the removal of two electrons from a neutral species.Diatomic dications corresponding to stable neutral species often decay quickly into two singly charged particles , due to the loss of electrons in bonding molecular orbitals...
of adamantane was obtained in solutions of superacid
Superacid
According to the classical definition superacid is an acid with an acidity greater than that of 100% pure sulfuric acid, which has a Hammett acidity function of −12. According to the modern definition, superacid is a medium, in which the chemical potential of the proton is higher than in pure...
s. It also has elevated stability due to the phenomenon called "three-dimensional aromaticity".
Reactions
Most reactions of adamantane occur via the 3-coordinated carbon sites and are described in the subsections below. The 2-coordinated, bridging carbon sites are much less reactive. They are involved in the reaction of adamantane with concentrated sulfuric acidSulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
which produces adamantanone.
The carbonyl group
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
of adamantanone allows further reactions via the bridging site. For example, adamantanone is the starting compound for obtaining such derivatives of adamantane as 2-adamantanecarbonitrile and 2-methyl-adamantane.
Bromination
Adamantane readily reacts with various reagents brominating compounds such as molecular bromineBromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
. The composition and the ratio of the reaction products depend on the reaction conditions and especially presence of catalyst s.
Boiling of adamantane with bromine results in a monosubstituted adamantane, 1-bromadamantane. Multiple substitution with bromine is achieved by adding a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
catalyst.
The rate of bromination is accelerated upon addition of Lewis acids and is unchanged by irradiation or addition of free radicals. This indicates that the reaction occurs via an ionic mechanism.
Fluorination
The first fluorinations of adamantane were conducted using 1-hydroxyadamantane and 1-aminoadamantane as initial compounds. Later, fluorination was achieved starting from adamantane itself. In all these cases, reaction proceeded via formation of adamantane cation which then interacted with fluorinated nucleophiles. Fluorination of adamantane with gaseous fluorineFluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
has also been reported.
Carboxylation
Carboxylation of adamantane was first reported in 1960, using formic acidFormic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
as a carboxylating agent and carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
as a solvent.
tert-butanol
Tert-Butanol
tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers of butanol. tert-Butanol is a clear liquid with a camphor-like odor. It is very soluble in water and miscible with ethanol and diethyl ether...
(t-BuOH) and sulfuric acid were added to generate adamantane cation; the cation was then carboxylated by carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
generated in situ in the interaction between the formic
Formic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
and sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
s. The fraction of carboxylated adamantane was 55-60%.1-Adamantanecarboxylic acid Organic Syntheses, Coll. Vol. 5, p. 20 (1973); Vol. 44, p. 1 (1964).
Hydroxylation
The simplest adamantane alcohol, 1-hydroxyadamantane, is readily formed by hydrolysis of 1-bromadamantane in aqueous solution of acetoneAcetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
. It can also be produced by ozonation of the adamantane:
Others
Adamantane interacts with benzeneBenzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
in the presence of Lewis acids, resulting in a Friedel–Crafts reaction. Aromatically substituted adamantane derivatives can be easily obtained starting from 1-hydroxyadamantane. In particular, the reaction with anisole
Anisole
Anisole, or methoxybenzene, is the organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances...
proceeds under normal conditions and does not require a catalyst.
Nitration of adamantane is a difficult reaction characterized by moderate yields. An important nitrogen-substituted drug amantadine
Amantadine
Amantadine is the organic compound known formally as 1-adamantylamine or 1-aminoadamantane. The molecule consists of adamantane backbone that has an amino group substituted at one of the four methyne positions. This pharmaceutical is sold under the name Symmetrel for use both as an antiviral and an...
can be prepared by reacting adamantane with bromine or nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
to give the bromide or nitroester at the 1- position. Reaction of either compound with acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
affords the acetamide, which is hydrolyzed to give 1-adamantylamine:

Uses
Adamantane itself enjoys few applications since it is merely an unfunctionalized hydrocarbonHydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
. It is used in some dry etching
Dry etching
Dry etching refers to the removal of material, typically a masked pattern of semiconductor material, by exposing the material to a bombardment of ions that dislodge portions of the material from the exposed surface...
masks and polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
formulations.
In solid-state NMR spectroscopy, adamantane is a common standard for chemical shift
Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
referencing. In dye laser
Dye laser
A dye laser is a laser which uses an organic dye as the lasing medium, usually as a liquid solution. Compared to gases and most solid state lasing media, a dye can usually be used for a much wider range of wavelengths. The wide bandwidth makes them particularly suitable for tunable lasers and...
s, adamantane may be used to extend the life of the gain medium; it cannot be photoionized under atmosphere because its absorption bands lie in the vacuum-ultraviolet region of the spectrum. Photoionization energies have been determined for adamantane as well as for several bigger diamondoids.
In medicine
All medical applications known so far involve not pure adamantane, but its derivatives. The first adamantane derivative used as a drug was amantadineAmantadine
Amantadine is the organic compound known formally as 1-adamantylamine or 1-aminoadamantane. The molecule consists of adamantane backbone that has an amino group substituted at one of the four methyne positions. This pharmaceutical is sold under the name Symmetrel for use both as an antiviral and an...
– first (1967) as an antiviral drug
Antiviral drug
Antiviral drugs are a class of medication used specifically for treating viral infections. Like antibiotics for bacteria, specific antivirals are used for specific viruses...
against various strains of flu and then to treat the Parkinson's disease
Parkinson's disease
Parkinson's disease is a degenerative disorder of the central nervous system...
. Other drugs among adamantane derivatives include memantine, rimantadine
Rimantadine
Rimantadine is an orally administered antiviral drug used to treat, and in rare cases prevent, influenzavirus A infection. When taken within one to two days of developing symptoms, rimantadine can shorten the duration and moderate the severity of influenza. Both rimantadine and the similar drug...
, dopamantin, tromantadine, vildagliptin and karmantadin. Polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s of adamantane have been patented as antiviral agents against HIV
HIV
Human immunodeficiency virus is a lentivirus that causes acquired immunodeficiency syndrome , a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive...
.
Potential technological applications
Some alkyl derivatives of adamantane have been used as a working fluid in hydraulic systemsHydraulic cylinder
A Hydraulic cylinder is a mechanical actuator that is used to give a unidirectional force through a unidirectional stroke. It has many applications, notably in engineering vehicles.- Operation :...
. Adamantane-based polymers might find application for coatings of touchscreen
Touchscreen
A touchscreen is an electronic visual display that can detect the presence and location of a touch within the display area. The term generally refers to touching the display of the device with a finger or hand. Touchscreens can also sense other passive objects, such as a stylus...
s, and there are prospects for using adamantane and its homologues in nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
. For example, the soft cage-like structure of adamantane solid allow incorporation of guest molecules, which can be released inside the human body upon breaking the matrix.
Adamantane analogues
Many molecules adopt adamantane-like cage structures. Those include phosphorus trioxidePhosphorus trioxide
Phosphorus trioxide is the chemical compound with the molecular formula P4O6. This compound was discovered by Neil G. Mehta . Although it should properly be named tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage...
P4O6, arsenic trioxide
Arsenic trioxide
Arsenic trioxide is the inorganic compound with the formula As2O3. This commercially important oxide of arsenic is the main precursor to other arsenic compounds, including organoarsenic compounds. Approximately 50,000 tonnes are produced annually...
As4O6, phosphorus pentoxide
Phosphorus pentoxide
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 . This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant.-Structure:...
P4O10 = (PO)4O6, phosphorus pentasulfide
Phosphorus pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value...
P4S10 = (PS)4S6, and hexamethylenetetramine C6N4H12 = N4(CH2)6. Particularly notorious is tetramethylenedisulfotetramine
Tetramethylenedisulfotetramine
Tetramethylenedisulfotetramine is an organic compound that is used as a rodenticide . It is an odorless, tasteless white powder that is slightly soluble in water, DMSO and acetone, and insoluble in methanol and ethanol. It is a sulfamide derivative...
, often shortened to "tetramine", a rodenticide banned in most countries for extreme toxicity to humans. The silicon analogue of adamantane, sila-adamantane, was synthesized in 2005.
Adamantane cages can be stacked together to produce higher diamondoids, such as diamantane (C14H20 – two fused adamantane cages), triamantane (C18H24), tetramantane (C22H28), pentamantane (C26H32), hexamantane (C26H30), etc. Their synthesis is similar to that of adamantane and like adamantane, they can also be extracted from petroleum, though at even much smaller yields.

