Limonene
Encyclopedia
Limonene is a colourless liquid hydrocarbon
classified as a cyclic terpene
. The more common D isomer possesses a strong smell of oranges
. It is used in chemical synthesis as a precursor to carvone
and as a renewably-based solvent in cleaning products.
Limonene takes its name from the lemon
, as the rind
of the lemon, like other citrus
fruits, contains considerable amounts of this compound, which contributes to their odor. Limonene is a chiral
molecule, and biological sources produce one enantiomer
: the principal industrial source, citrus fruit, contains D-limonene ((+)-limonene), which is the (R)-enantiomer
(CAS number
5989-27-5, EINECS number 227-813-5). Racemic
limonene is known as dipentene. D-Limonene is obtained commercially from citrus fruits through two primary methods: centrifugal separation or steam distillation.
and can be distilled without decomposition, although at elevated temperatures it cracks
to form isoprene
. It oxidizes easily in moist air to produce carveol
, carvone
, and limonene oxide. With sulfur, it undergoes dehydrogenation to p-cymene
.
Limonene occurs naturally as the (R)-enantiomer
, but racemizes to dipentene at 300 °C. When warmed with mineral acid
, limonene isomerizes to the conjugated diene
α-terpinene
(which can also easily be converted to p-cymene). Evidence for this isomerization includes the formation of Diels-Alder
adducts between α-terpinene adducts and maleic anhydride
.
It is possible to effect reaction at one of the double bonds selectively. Anhydrous hydrogen chloride
reacts preferentially at the disubstituted alkene, whereas epoxidation with MCPBA
occurs at the trisubstituted alkene.
In another synthetic method Markovnikov addition
of trifluoroacetic acid
followed by hydrolysis
of the acetate gives terpineol
.
, via cyclization of a neryl
carbocation
or its equivalent as shown. The final step involves loss of a proton from the cation to form the alkene
.
. The three step reaction begins with the regioselective addition of nitrosyl chloride across the trisubstituted double bond. This species is then converted to the oxime
with base, and the hydroxylamine is removed to give the ketone-containing carvone.
Limonene is common in cosmetic
products. As the main odor constituent of citrus
(plant family Rutaceae
), D-limonene is used in food manufacturing and some medicines, e.g. as a flavoring to mask the bitter taste of alkaloids, and as a fragrant in perfumery; it is also used as botanical insecticide
., particularly the (R)-(+)-enantiomer
is most active as an insecticide
. It is added to cleaning products such as hand cleansers to give a lemon-orange fragrance (see orange oil
). In contrast, L-limonene has a pine
y, turpentine
-like odor.
In natural and alternative medicine, D-limonene is marketed to relieve gastroesophageal reflux disease
and heartburn
.
Limonene is increasingly being used as a solvent
for cleaning purposes, such as the removal of oil from machine parts, as it is produced from a renewable source (citrus oil, as a byproduct of orange juice
manufacture). It also serves as a paint stripper when applied to painted wood and is also useful as a fragrant alternative to turpentine
. Limonene is also used as a solvent in some model airplane glues. All-natural commercial air fresheners, with air propellants, containing limonene are used by philatelists to remove self-adhesive postage stamps from envelope paper.
As it is combustible, limonene has also been considered as a biofuel
.
Limonene can be used to dissolve polystyrene, and is a more ecologically friendly substitute for acetone.
In preparing tissues for histology
or histopathology, D-limonene is often used as a less toxic substitute for xylene when clearing dehydrated specimens. Clearing agents are liquids miscible with alcohols (such as ethanol or isopropanol) and with melted paraffin wax, in which specimens are embedded to facilitate cutting of thin sections for microscopy.
showed that 3% were sensitized to limonene.
Although high doses have been shown to cause renal cancer
in male rats, limonene is considered by some researchers to be a potential chemopreventive
agent
with value as a dietary anti-cancer tool in humans. There is no evidence for carcinogen
icity or genotoxic
ity in humans. The IARC
classifies D-limonene as a Group 3 carcinogen: not classifiable as to its carcinogenicity to humans.
No information is available on the health effects of inhalation exposure to D-limonene in humans, and no long-term inhalation studies have been conducted in laboratory animals.
D-Limonene is biodegradable, but due to its low flash point, it must be treated as hazardous waste for disposal.
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
classified as a cyclic terpene
Terpene
Terpenes are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers, though also by some insects such as termites or swallowtail butterflies, which emit terpenes from their osmeterium. They are often strong smelling and thus may have had a protective...
. The more common D isomer possesses a strong smell of oranges
Orange (fruit)
An orange—specifically, the sweet orange—is the citrus Citrus × sinensis and its fruit. It is the most commonly grown tree fruit in the world....
. It is used in chemical synthesis as a precursor to carvone
Carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway and dill.-Stereoisomerism and odor:...
and as a renewably-based solvent in cleaning products.
Limonene takes its name from the lemon
Lemon
The lemon is both a small evergreen tree native to Asia, and the tree's ellipsoidal yellow fruit. The fruit is used for culinary and non-culinary purposes throughout the world – primarily for its juice, though the pulp and rind are also used, mainly in cooking and baking...
, as the rind
Peel (fruit)
Peel, also known as rind or skin, is the outer protective layer of a fruit or vegetable which could be peeled off. The rind is usually the botanical exocarp, but the term exocarp does also include the hard cases of nuts, which are not named peels since they are not peeled off by hand or peeler, but...
of the lemon, like other citrus
Citrus
Citrus is a common term and genus of flowering plants in the rue family, Rutaceae. Citrus is believed to have originated in the part of Southeast Asia bordered by Northeastern India, Myanmar and the Yunnan province of China...
fruits, contains considerable amounts of this compound, which contributes to their odor. Limonene is a chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
molecule, and biological sources produce one enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
: the principal industrial source, citrus fruit, contains D-limonene ((+)-limonene), which is the (R)-enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
(CAS number
CAS registry number
CAS Registry Numbersare unique numerical identifiers assigned by the "Chemical Abstracts Service" toevery chemical described in the...
5989-27-5, EINECS number 227-813-5). Racemic
Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
limonene is known as dipentene. D-Limonene is obtained commercially from citrus fruits through two primary methods: centrifugal separation or steam distillation.
Chemical reactions
Limonene is a relatively stable terpeneTerpene
Terpenes are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers, though also by some insects such as termites or swallowtail butterflies, which emit terpenes from their osmeterium. They are often strong smelling and thus may have had a protective...
and can be distilled without decomposition, although at elevated temperatures it cracks
Cracking (chemistry)
In petroleum geology and chemistry, cracking is the process whereby complex organic molecules such as kerogens or heavy hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products...
to form isoprene
Isoprene
Isoprene , or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=CCH=CH2. Under standard conditions it is a colorless liquid...
. It oxidizes easily in moist air to produce carveol
Carveol
Carveol is a natural unsaturated, monocyclic monoterpenoid alcohol that is a constituent of spearmint essential oil in the form of cis--carveol. It is a colorless fluid soluble in water and oils, but insoluble in water and has an odor and flavor that resemble those of spearmint and caraway...
, carvone
Carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway and dill.-Stereoisomerism and odor:...
, and limonene oxide. With sulfur, it undergoes dehydrogenation to p-cymene
Cymene
Cymene, or p-cymene, is a naturally occurring aromatic organic compound. It is classified as a hydrocarbon related to a monoterpene. Its structure consists of a benzene ring para-substituted with a methyl group and an isopropyl group...
.
Limonene occurs naturally as the (R)-enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
, but racemizes to dipentene at 300 °C. When warmed with mineral acid
Mineral acid
A mineral acid is an acid derived from one or more inorganic compounds. A mineral acid is not organic and all mineral acids release hydrogen ions when dissolved in water.-Characteristics:...
, limonene isomerizes to the conjugated diene
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
α-terpinene
Terpinene
The terpinenes are three isomeric hydrocarbons that are classified as terpenes. They each have the same molecular formula and carbon framework, but they differ in the position of carbon-carbon double bonds. α-Terpinene has been isolated from cardamom and marjoram oils, and from other natural...
(which can also easily be converted to p-cymene). Evidence for this isomerization includes the formation of Diels-Alder
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
adducts between α-terpinene adducts and maleic anhydride
Maleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
.
It is possible to effect reaction at one of the double bonds selectively. Anhydrous hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
reacts preferentially at the disubstituted alkene, whereas epoxidation with MCPBA
Meta-Chloroperoxybenzoic acid
meta-Chloroperoxybenzoic acid is a peroxycarboxylic acid used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling...
occurs at the trisubstituted alkene.
In another synthetic method Markovnikov addition
Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule is an observation based on Zaitsev's rule. It was formulated by the Russian chemist Vladimir Vasilevich Markovnikov in 1870....
of trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
followed by hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the acetate gives terpineol
Terpineol
Terpineol is a naturally occurring monoterpene alcohol that has been isolated from a variety of sources such as cajuput oil, pine oil, and petitgrain oil. There are three isomers, alpha-, beta-, and gamma-terpineol, the last two differing only by the location of the double bond...
.
Biosynthesis
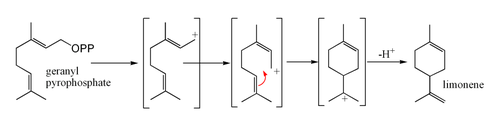
Limonene is formed from geranyl pyrophosphateGeranyl pyrophosphate
Geranyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of farnesyl pyrophosphate, geranylgeranyl pyrophosphate, cholesterol, terpenes and terpenoids....
, via cyclization of a neryl
Nerol
Nerol is a monoterpene found in many essential oils such as lemongrass and hops. It was originally isolated from neroli oil, hence its name. This colourless liquid is used in perfumery. Like geraniol, nerol has a sweet rose odor but it is considered to be fresher.Isomeric with nerol is geraniol,...
carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
or its equivalent as shown. The final step involves loss of a proton from the cation to form the alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
.

Uses
The most widely practiced conversion of limonene is to carvoneCarvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway and dill.-Stereoisomerism and odor:...
. The three step reaction begins with the regioselective addition of nitrosyl chloride across the trisubstituted double bond. This species is then converted to the oxime
Oxime
An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=NOH, where R1 is an organic side chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds...
with base, and the hydroxylamine is removed to give the ketone-containing carvone.
Limonene is common in cosmetic
Cosmetics
Cosmetics are substances used to enhance the appearance or odor of the human body. Cosmetics include skin-care creams, lotions, powders, perfumes, lipsticks, fingernail and toe nail polish, eye and facial makeup, towelettes, permanent waves, colored contact lenses, hair colors, hair sprays and...
products. As the main odor constituent of citrus
Citrus
Citrus is a common term and genus of flowering plants in the rue family, Rutaceae. Citrus is believed to have originated in the part of Southeast Asia bordered by Northeastern India, Myanmar and the Yunnan province of China...
(plant family Rutaceae
Rutaceae
Rutaceae, commonly known as the rue or citrus family, is a family of flowering plants, usually placed in the order Sapindales.Species of the family generally have flowers that divide into four or five parts, usually with strong scents...
), D-limonene is used in food manufacturing and some medicines, e.g. as a flavoring to mask the bitter taste of alkaloids, and as a fragrant in perfumery; it is also used as botanical insecticide
Insecticide
An insecticide is a pesticide used against insects. They include ovicides and larvicides used against the eggs and larvae of insects respectively. Insecticides are used in agriculture, medicine, industry and the household. The use of insecticides is believed to be one of the major factors behind...
., particularly the (R)-(+)-enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
is most active as an insecticide
Insecticide
An insecticide is a pesticide used against insects. They include ovicides and larvicides used against the eggs and larvae of insects respectively. Insecticides are used in agriculture, medicine, industry and the household. The use of insecticides is believed to be one of the major factors behind...
. It is added to cleaning products such as hand cleansers to give a lemon-orange fragrance (see orange oil
Orange oil
Orange oil is an essential oil produced by cells inside the rind of an orange fruit. In contrast to most essential oils, it is extracted as a by-product of orange juice production by centrifugation, producing a cold-pressed oil...
). In contrast, L-limonene has a pine
Pine
Pines are trees in the genus Pinus ,in the family Pinaceae. They make up the monotypic subfamily Pinoideae. There are about 115 species of pine, although different authorities accept between 105 and 125 species.-Etymology:...
y, turpentine
Turpentine
Turpentine is a fluid obtained by the distillation of resin obtained from trees, mainly pine trees. It is composed of terpenes, mainly the monoterpenes alpha-pinene and beta-pinene...
-like odor.
In natural and alternative medicine, D-limonene is marketed to relieve gastroesophageal reflux disease
Gastroesophageal reflux disease
Gastroesophageal reflux disease , gastro-oesophageal reflux disease , gastric reflux disease, or acid reflux disease is chronic symptoms or mucosal damage caused by stomach acid coming up from the stomach into the esophagus...
and heartburn
Heartburn
Heartburn, also known as pyrosis or acid indigestion is a burning sensation in the chest, just behind the breastbone or in the epigastrium...
.
Limonene is increasingly being used as a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
for cleaning purposes, such as the removal of oil from machine parts, as it is produced from a renewable source (citrus oil, as a byproduct of orange juice
Orange juice
Orange juice is a popular beverage made from oranges. It is made by extraction from the fresh fruit, by desiccation and subsequent reconstitution of dried juice, or by concentration of the juice and the subsequent addition of water to the concentrate...
manufacture). It also serves as a paint stripper when applied to painted wood and is also useful as a fragrant alternative to turpentine
Turpentine
Turpentine is a fluid obtained by the distillation of resin obtained from trees, mainly pine trees. It is composed of terpenes, mainly the monoterpenes alpha-pinene and beta-pinene...
. Limonene is also used as a solvent in some model airplane glues. All-natural commercial air fresheners, with air propellants, containing limonene are used by philatelists to remove self-adhesive postage stamps from envelope paper.
As it is combustible, limonene has also been considered as a biofuel
Biofuel
Biofuel is a type of fuel whose energy is derived from biological carbon fixation. Biofuels include fuels derived from biomass conversion, as well as solid biomass, liquid fuels and various biogases...
.
Limonene can be used to dissolve polystyrene, and is a more ecologically friendly substitute for acetone.
In preparing tissues for histology
Histology
Histology is the study of the microscopic anatomy of cells and tissues of plants and animals. It is performed by examining cells and tissues commonly by sectioning and staining; followed by examination under a light microscope or electron microscope...
or histopathology, D-limonene is often used as a less toxic substitute for xylene when clearing dehydrated specimens. Clearing agents are liquids miscible with alcohols (such as ethanol or isopropanol) and with melted paraffin wax, in which specimens are embedded to facilitate cutting of thin sections for microscopy.
Safety
Limonene and its oxidation products are skin and respiratory irritants, and limonene-1,2-oxide (formed by aerial oxidation) is a known skin sensitizer. Most reported cases of irritation have involved long-term industrial exposure to the pure compound, e.g. during degreasing or the preparation of paints. However a study of patients presenting dermatitisDermatitis
-Etymology:Dermatitis derives from Greek derma "skin" + -itis "inflammation" and genetic disorder.-Terminology:There are several different types of dermatitis. The different kinds usually have in common an allergic reaction to specific allergens. The term may describe eczema, which is also called...
showed that 3% were sensitized to limonene.
Although high doses have been shown to cause renal cancer
Renal cell carcinoma
Renal cell carcinoma is a kidney cancer that originates in the lining of the proximal convoluted tubule, the very small tubes in the kidney that filter the blood and remove waste products. RCC is the most common type of kidney cancer in adults, responsible for approximately 80% of cases...
in male rats, limonene is considered by some researchers to be a potential chemopreventive
Chemoprophylaxis
Chemoprophylaxis refers to the administration of a medication for the purpose of preventing disease or infection. Antibiotics, for example, may be administered to patients with disorders of immune system function to prevent bacterial infections...
agent
with value as a dietary anti-cancer tool in humans. There is no evidence for carcinogen
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that is an agent directly involved in causing cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes...
icity or genotoxic
Genotoxic
In genetics, genotoxicity describes a deleterious action on a cell's genetic material affecting its integrity. This includes both certain chemical compounds and certain types of radiation....
ity in humans. The IARC
International Agency for Research on Cancer
The International Agency for Research on Cancer is an intergovernmental agency forming part of the World Health Organisation of the United Nations....
classifies D-limonene as a Group 3 carcinogen: not classifiable as to its carcinogenicity to humans.
No information is available on the health effects of inhalation exposure to D-limonene in humans, and no long-term inhalation studies have been conducted in laboratory animals.
D-Limonene is biodegradable, but due to its low flash point, it must be treated as hazardous waste for disposal.

