Diamondoid
Encyclopedia
A diamondoid, in the context of building materials for nanotechnology
components, most generally refers to structures that resemble diamond
in a broad sense: namely, strong, stiff structures containing dense, 3-D networks of covalent bond
s, formed chiefly from first and second row atoms with a valence
of three or more. Examples of diamondoid structures would include crystalline diamond
, sapphire
, and other stiff structures similar to diamond but with various atom substitutions which might include N, O, Si, S, and so forth. Sp²-hybridized
carbon structures that – in contrast to sp³-hybridized
carbon in diamond
– arrange in planar sheets ("graphene
" sheets) are sometimes also included in the class of diamondoid materials for nanotechnology
, e.g., graphite
, carbon nanotubes consisting of sheets of carbon
atoms rolled into tubes, spherical buckyballs and other graphene
structures.
cage molecule known as adamantane
(C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. These species are of interest as molecular approximations of the cubic diamond framework, terminated with C-H bonds. Cyclohexamantane may be thought of as a nanometer-sized diamond of approximately 5.6 * 10−22 grams.
 Examples include:
Examples include:
One tetramantane isomer is the largest ever diamondoid prepared by organic synthesis
. The first ever isolation of a wide range of diamondoids from petroleum took place in the following steps : a vacuum distillation
above 345 °C, the equivalent atmospheric boiling point, then pyrolysis
at 400 to 450 °C in order to remove all non-diamondoid compounds and then a series of HPLC
separation techniques.
In one study a tetramantane compound is fitted with thiol
groups at the bridgehead positions. This allows their anchorage to a gold surface and formation of self-assembled monolayer
s (diamond-on-gold).
Organic chemistry of diamondoids even extends to pentamantane. The medial position (base) in this molecule is calculated to yield a more favorable carbocation
than the apical position (top) and simple bromination of pentamane 1 with bromine exclusively gives the medial bromo derivative 2 which on hydrolysis in water / DMF
forms the alcohol
3.
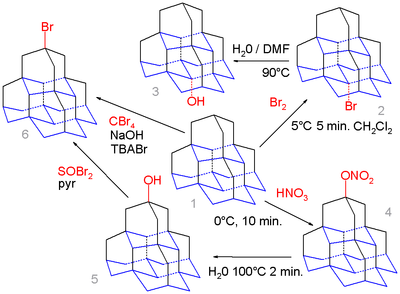 In contrast nitroxylation of 1 with nitric acid
In contrast nitroxylation of 1 with nitric acid
gives the apical nitrate
4 as an intermediate which is hydrolyzed to the apical alcohol
5 due to the higher steric demand of the active electrophilic NO2 - HNO3+ species. This alcohol can react with thionyl bromide
to the bromide 6 and in a series of steps (not shown) to the corresponding thiol
. Pentamantane can also react with tetrabromomethane
and tetra-n-butylammonium
Bromide (TBABr) in a free radical reaction
to the bromide but without selectivity.
s present.
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
components, most generally refers to structures that resemble diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
in a broad sense: namely, strong, stiff structures containing dense, 3-D networks of covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
s, formed chiefly from first and second row atoms with a valence
Valence (chemistry)
In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
of three or more. Examples of diamondoid structures would include crystalline diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
, sapphire
Sapphire
Sapphire is a gemstone variety of the mineral corundum, an aluminium oxide , when it is a color other than red or dark pink; in which case the gem would instead be called a ruby, considered to be a different gemstone. Trace amounts of other elements such as iron, titanium, or chromium can give...
, and other stiff structures similar to diamond but with various atom substitutions which might include N, O, Si, S, and so forth. Sp²-hybridized
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
carbon structures that – in contrast to sp³-hybridized
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
carbon in diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
– arrange in planar sheets ("graphene
Graphene
Graphene is an allotrope of carbon, whose structure is one-atom-thick planar sheets of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice. The term graphene was coined as a combination of graphite and the suffix -ene by Hanns-Peter Boehm, who described single-layer...
" sheets) are sometimes also included in the class of diamondoid materials for nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
, e.g., graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
, carbon nanotubes consisting of sheets of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atoms rolled into tubes, spherical buckyballs and other graphene
Graphene
Graphene is an allotrope of carbon, whose structure is one-atom-thick planar sheets of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice. The term graphene was coined as a combination of graphite and the suffix -ene by Hanns-Peter Boehm, who described single-layer...
structures.
Chemistry
In the context of classical chemistry, "diamondoid" refers to variants of the carbonCarbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
cage molecule known as adamantane
Adamantane
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid...
(C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. These species are of interest as molecular approximations of the cubic diamond framework, terminated with C-H bonds. Cyclohexamantane may be thought of as a nanometer-sized diamond of approximately 5.6 * 10−22 grams.

- AdamantaneAdamantaneAdamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C10H16, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consist of three cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid...
(C10H16) - IceaneIceaneIceane is a saturated polycyclic hydrocarbon with formula C12H18. It has a cage-like molecular structure, whose carbon skeleton can be viewed as three fused cyclohexane rings in the "boat" conformation; or as two such rings in the "chair" conformation, connected by three parallel bonds.The name...
(C12H18) - BC-8 (C14H20)
- Diamantane (C14H20) also diadamantane, two face-fused cages
- Triamantane (C18H24), also triadamantane. Diamantane has 4 identical faces available for anchoring a new C4H4 unit.
- Isotetramantane (C22H28). Triamantane has 8 faces on to which a new C4H4 unit can be added resulting in 4 isomerIsomerIn chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s. One of these isomers displays a helical twist and is therefore prochiralProchiralIn stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step.If two identical substituents are attached to a sp3-hybridized atom, the descriptors pro-R and pro-S are used to distinguish between the two...
. The P and M enantiomerEnantiomerIn chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s have been separated. - Pentamantane has 9 isomers with chemical formula C26H32 and one more pentamantane exists with chemical formula C25H30
- Cyclohexamantane (C26H30)
- Super-adamantane (C30H36)
- Basic beryllium acetateBasic beryllium acetateBasic beryllium acetate is the chemical compound with the formula Be4O6. Although this compound has no applications and has been only lightly studied, it adopts a distinctive structure. "Basic acetates" consist of an ensemble of metal atoms, a central oxide atom, and an exterior of acetate groups...
Be4O(O2CCH3)6
One tetramantane isomer is the largest ever diamondoid prepared by organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. The first ever isolation of a wide range of diamondoids from petroleum took place in the following steps : a vacuum distillation
Vacuum distillation
Vacuum distillation is a method of distillation whereby the pressure above the liquid mixture to be distilled is reduced to less than its vapor pressure causing evaporation of the most volatile liquid...
above 345 °C, the equivalent atmospheric boiling point, then pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
at 400 to 450 °C in order to remove all non-diamondoid compounds and then a series of HPLC
High-performance liquid chromatography
High-performance liquid chromatography , HPLC, is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.HPLC typically utilizes different types of stationary...
separation techniques.
In one study a tetramantane compound is fitted with thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
groups at the bridgehead positions. This allows their anchorage to a gold surface and formation of self-assembled monolayer
Self-assembled monolayer
A self assembled monolayer is an organized layer of amphiphilic molecules in which one end of the molecule, the “head group” shows a specific, reversible affinity for a substrate...
s (diamond-on-gold).
Organic chemistry of diamondoids even extends to pentamantane. The medial position (base) in this molecule is calculated to yield a more favorable carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
than the apical position (top) and simple bromination of pentamane 1 with bromine exclusively gives the medial bromo derivative 2 which on hydrolysis in water / DMF
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
forms the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
3.

Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
gives the apical nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
4 as an intermediate which is hydrolyzed to the apical alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
5 due to the higher steric demand of the active electrophilic NO2 - HNO3+ species. This alcohol can react with thionyl bromide
Thionyl bromide
Thionyl bromide is the chemical compound SOBr2. It is less stable and less widely used than its chloride analogue, thionyl chloride. It is prepared by the action of hydrogen bromide on thionyl chloride, a characteristic reaction where a stronger acid is converted to a weaker acid:Thionyl bromide is...
to the bromide 6 and in a series of steps (not shown) to the corresponding thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
. Pentamantane can also react with tetrabromomethane
Tetrabromomethane
Tetrabromomethane, CBr4, also known as carbon tetrabromide, is a carbon bromide. Both names are acceptable under IUPAC nomenclature, depending on whether it is considered as an organic or an inorganic compound.- Physical properties :...
and tetra-n-butylammonium
Quaternary ammonium cation
Quaternary ammonium cations, also known as quats, are positively charged polyatomic ions of the structure NR4+, R being an alkyl group or an aryl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged,...
Bromide (TBABr) in a free radical reaction
Free radical reaction
A free radical reaction is any chemical reaction involving free radicals. This reaction type is abundant in organic reactions.Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical by Moses Gomberg and the lead-mirror experiment described by...
to the bromide but without selectivity.
Origin and occurrence of diamondoids
Diamondoids are found in mature high-temperature petroleum fluids (volatile oils, condensates and wet gases). These fluids can have up to a spoonful of diamondoids per gallon (about 3.78 liters). A review by Mello and Moldowan in 2005 showed that although the carbon in diamonds is not biological in origin, the diamondoids found in petroleum are composed of carbon from biological sources. This was determined by comparing the ratios of carbon isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s present.

