
Tert-Butanol
Encyclopedia
tert-Butanol, or 2-methyl-2-propanol, is the simplest tertiary alcohol. It is one of the four isomers
of butanol
. tert-Butanol is a clear liquid (or a colorless solid, depending on the ambient temperature) with a camphor
-like odor. It is very soluble in water and miscible with ethanol
and diethyl ether
. It is unique among the isomers of butanol because it tends to be a solid at room temperature, with a melting point slightly above 25 °C.
production. It can also be produced by the catalytic hydration
of isobutylene
.
booster for gasoline
, as an oxygenate
gasoline additive
, and as an intermediate in the synthesis of other chemical commodities such as MTBE, ETBE
, TBHP, other flavors and perfumes.
When tert-butanol is deprotonated with a strong base
, the product is an alkoxide
anion. In this case, it is tert-butoxide. For example, the commonly used organic reagent potassium tert-butoxide
is prepared by refluxing dry tert-butanol with potassium
metal.
The tert-butoxide species is itself useful as a strong, non-nucleophilic base in organic chemistry. It is able to abstract acidic protons from the substrate molecule readily, but its steric bulk inhibits the group from participating in nucleophilic substitution, such as in a Williamson ether synthesis
or an SN2 reaction.
to form tert-butyl chloride and water via an SN1
mechanism.
The overall reaction, therefore, is:
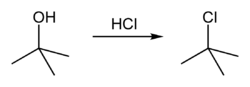
Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the Step 2 allows the SN1 mechanism to be followed. Primary alcohols generally undergo an SN2 mechanism because the relative stability of a primary carbocation intermediate is very low. The tertiary carbocation in this case is stabilized through hyperconjugation where the neighboring C–H sigma bonds donate electrons into the empty p-orbital of the carbocation.
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
of butanol
Butanol
Butanol or butyl alcohol can refer to any of the four isomeric alcohols of formula C4H9OH:*n-Butanol, butan-1-ol, 1-butanol, n-butyl alcohol;*Isobutanol, 2-methylpropan-1-ol, isobutyl alcohol;...
. tert-Butanol is a clear liquid (or a colorless solid, depending on the ambient temperature) with a camphor
Camphor
Camphor is a waxy, white or transparent solid with a strong, aromatic odor. It is a terpenoid with the chemical formula C10H16O. It is found in wood of the camphor laurel , a large evergreen tree found in Asia and also of Dryobalanops aromatica, a giant of the Bornean forests...
-like odor. It is very soluble in water and miscible with ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. It is unique among the isomers of butanol because it tends to be a solid at room temperature, with a melting point slightly above 25 °C.
Preparation
tert-Butanol is derived commercially from isobutane as a co-product of propylene oxidePropylene oxide
Propylene oxide is an organic compound with the molecular formula CH3CHCH2O. This colourless volatile liquid is produced on a large scale industrially, its major application being its use for the production of polyether polyols for use in making polyurethane plastics...
production. It can also be produced by the catalytic hydration
Hydration reaction
In organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous...
of isobutylene
Isobutylene
Isobutylene is a hydrocarbon of significant industrial importance. It is a four-carbon branched alkene , one of the four isomers of butylene. At standard temperature and pressure it is a colorless flammable gas.-Uses:...
.
Applications
tert-Butanol is used as a solvent, as a denaturant for ethanol, as an ingredient in paint removers, as an octaneOctane rating
Octane rating or octane number is a standard measure of the anti-knock properties of a motor or aviation fuel. The higher the octane number, the more compression the fuel can withstand before detonating...
booster for gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
, as an oxygenate
Oxygenate
Oxygenated chemical compounds contain oxygen as a part of their chemical structure. The term usually refers to oxygenated fuels. Oxygenates are usually employed as gasoline additives to reduce carbon monoxide that is created during the burning of the fuel....
gasoline additive
Gasoline additive
Gasoline additives increase gasoline's octane rating or act as corrosion inhibitors or lubricants, thus allowing the use of higher compression ratios for greater efficiency and power, however some carry heavy environmental risks...
, and as an intermediate in the synthesis of other chemical commodities such as MTBE, ETBE
ETBE
Ethyl tert-butyl ether is commonly used as an oxygenate gasoline additive in the production of gasoline from crude oil. ETBE offers equal or greater air quality benefits than ethanol, while being technically and logistically less challenging...
, TBHP, other flavors and perfumes.
Chemistry
As a tertiary alcohol, tert-butanol is more stable to oxidation and less reactive than the other isomers of butanol.When tert-butanol is deprotonated with a strong base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
, the product is an alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
anion. In this case, it is tert-butoxide. For example, the commonly used organic reagent potassium tert-butoxide
Potassium tert-butoxide
Potassium tert-butoxide is the chemical compound with the formula 3COK. This colourless solid is a strong base useful in organic synthesis. It exists as a tetrameric cubane-like cluster...
is prepared by refluxing dry tert-butanol with potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
metal.
- K + tBuOH → tBuO−K+ + 1/2 H2
The tert-butoxide species is itself useful as a strong, non-nucleophilic base in organic chemistry. It is able to abstract acidic protons from the substrate molecule readily, but its steric bulk inhibits the group from participating in nucleophilic substitution, such as in a Williamson ether synthesis
Williamson ether synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and an alcohol. This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction...
or an SN2 reaction.
Conversion to alkyl halide
tert-Butanol reacts with hydrogen chlorideHydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
to form tert-butyl chloride and water via an SN1
SN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
mechanism.
The overall reaction, therefore, is:

Because tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the Step 2 allows the SN1 mechanism to be followed. Primary alcohols generally undergo an SN2 mechanism because the relative stability of a primary carbocation intermediate is very low. The tertiary carbocation in this case is stabilized through hyperconjugation where the neighboring C–H sigma bonds donate electrons into the empty p-orbital of the carbocation.

