
Flavin
Encyclopedia
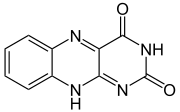
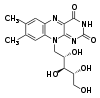
Pteridine
Pteridine is a chemical compound composed of fused pyrimidine and pyrazine rings. A pteridine is also a group of heterocyclic compounds containing a wide variety of substitutions on this structure. Pterins and flavins are classes of substituted pteridines that have important biological...
, formed by the tricyclic heteronuclear organic ring isoalloxazine. The biochemical source is the vitamin riboflavin
Riboflavin
Riboflavin, also known as vitamin B2 or additive E101, is an easily absorbed micronutrient with a key role in maintaining health in humans and animals. It is the central component of the cofactors FAD and FMN, and is therefore required by all flavoproteins. As such, vitamin B2 is required for a...
. The flavin moiety
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
is often attached with an adenosine diphosphate
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
to form flavin adenine dinucleotide (FAD), and, in other circumstances, is found as flavin mononucleotide
Flavin mononucleotide
Flavin mononucleotide , or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors...
(or FMN), a phosphorylated form of riboflavin
Riboflavin
Riboflavin, also known as vitamin B2 or additive E101, is an easily absorbed micronutrient with a key role in maintaining health in humans and animals. It is the central component of the cofactors FAD and FMN, and is therefore required by all flavoproteins. As such, vitamin B2 is required for a...
. It is in one or the other of these forms that flavin is present as a prosthetic group in flavoproteins.
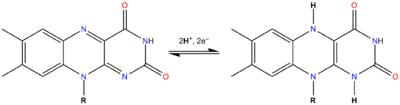
The flavin group is capable of undergoing oxidation-reduction reactions, and can accept either one electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
in a two-step process or two electrons at once. Reduction is made with the addition of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atoms to specific nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
atoms on the isoalloxazine ring system:
Aqueous solution
An aqueous solution is a solution in which the solvent is water. It is usually shown in chemical equations by appending aq to the relevant formula, such as NaCl. The word aqueous means pertaining to, related to, similar to, or dissolved in water...
, flavins are yellow-coloured when oxidized, taking a red colour in the semi-reduced anionic state or blue in the neutral (semiquinone
Semiquinone
Semiquinone is a free radical resulting from the removal of one hydrogen atom with its electron during the process of dehydrogenation of a hydroquinone to quinone or alternatively the addition of a single H atom to a quinone....
) state, and colourless when totally reduced. The oxidized and reduced forms are in fast equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
with the semiquinone (radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
) form, shifted against the formation of the radical:
-
- Flox + FlredH2 ⇌ FlH•
where Flox is the oxidized flavin, FlredH2 the reduced flavin (upon addition of two hydrogen atoms) and FlH• the semiquinone form (addition of one hydrogen atom).
In the form of FADH2, it is one of the cofactors that can transfer electrons to the electron transfer chain.
Photoreduction
Both free and protein-bound flavins are photoreducible, that is, able to be reduced by lightLight
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
, in a mechanism mediated by several organic compounds, such as some aminoacids, carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s and amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s.
FAD

Ferredoxin-NADP+ reductase
In enzymology, a ferredoxin-NADP reductase abbreviated FNR, is an enzyme that catalyzes the chemical reactionThe 3 substrates of this enzyme are reduced ferredoxin, NADP, and H, whereas its two products are oxidized ferredoxin and NADPH...
, monoamine oxidase
Monoamine oxidase
L-Monoamine oxidases are a family of enzymes that catalyze the oxidation of monoamines. They are found bound to the outer membrane of mitochondria in most cell types in the body. The enzyme was originally discovered by Mary Bernheim in the liver and was named tyramine oxidase...
, D-amino acid oxidase
D-amino acid oxidase
D-amino acid oxidase is a peroxisomal enzyme containing FAD as cofactor that is expressed in a wide range of species from yeasts to human. It is not present in bacteria or in plants...
, glucose oxidase
Glucose oxidase
The glucose oxidase enzyme is an oxido-reductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. In cells, it aids in breaking the sugar down into its metabolites....
, xanthine oxidase
Xanthine oxidase
Xanthine oxidase Xanthine oxidase Xanthine oxidase (XO (sometimes 'XAO'), a form of xanthine oxidoreductase that generates reactive oxygen species. Is an enzyme that catalyzes the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid...
, and acyl CoA dehydrogenase
Acyl CoA dehydrogenase
Acyl-CoA dehydrogenases are a class of enzymes that function to catalyze the initial step in each cycle of fatty acid β -oxidation in the mitochondria of cells. Their action results in the introduction of a trans double-bond between C2 and C3 of the acyl-CoA thioester substrate...
.
FADH/FADH2
FADH and FADH2 are reducedRedox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
forms of FAD. FADH2 is produced as a prosthetic group in succinate dehydrogenase, an enzyme involved in the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
. In oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
, two molecules of FADH2 typically yield 1.5 ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
each, or three ATP combined.
FMN
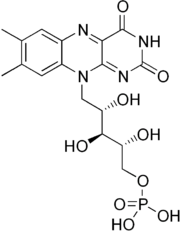
Flavin mononucleotide
Flavin mononucleotide , or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin by the enzyme riboflavin kinase and functions as prosthetic group of various oxidoreductases including NADH dehydrogenase as well as cofactor in biological blue-light photo receptors...
is a prosthetic group found in, among other proteins, NADH dehydrogenase
NADH dehydrogenase
NADH dehydrogenase is an enzyme located in the inner mitochondrial membrane that catalyzes the transfer of electrons from NADH to coenzyme Q...
, E.coli nitroreductase
E. coli nitroreductase
E. coli nitroreductase is a flavoprotein found in the bacteria Escherichia coli. It catalyses the reduction of nitro groups in a wide range of substrates, to produce the corresponding hydroxylamine. Although its role in vivo is unclear, it has been identified as useful in the metabolism of a number...
and old yellow enzyme.

