
Gas volume
Encyclopedia
In thermodynamics
, the volume of a system
is an important extensive parameter for describing its thermodynamic state
. The specific volume, an intensive property, is the system's volume per unit of mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure
and temperature
. For example, volume is related to the pressure
and temperature
of an ideal gas
by the ideal gas law
.
The physical volume of a system may or may not coincide with a control volume
used to analyze the system.
, or may be used to produce work. An isochoric process
however operates at a constant-volume, thus no work can be produced. Many other thermodynamic processes will result in a change in volume. A polytropic process, in particular, causes changes to the system so that the quantity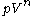 is constant (where
is constant (where 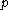 is pressure,
is pressure, 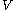 is volume, and
is volume, and  is the polytropic index, a constant). Note that for specific polytropic indexes a polytropic process will be equivalent to a constant-property process. For instance, for very large values of
is the polytropic index, a constant). Note that for specific polytropic indexes a polytropic process will be equivalent to a constant-property process. For instance, for very large values of  approaching infinity, the process becomes constant-volume.
approaching infinity, the process becomes constant-volume.
Gases are compressible, thus their volumes (and specific volumes) may be subject to change during thermodynamic processes. Fluids, however, are nearly incompressible, thus their volumes can be often taken as constant. In general, compressibility is defined as the relative volume change of a fluid or solid as a response to a pressure, and may be determined for substances in any phase. Similarly, thermal expansion
is the tendency of matter to change in volume in response to a change in temperature.
Many thermodynamic cycle
s are made up of varying processes, some which maintain a constant volume and some which do not. A vapor-compression refrigeration
cycle, for example, follows a sequence where the refrigerant fluid transitions between the liquid and vapor states of matter.
Typical units for volume are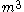 (cubic meters),
(cubic meters), 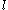 (liter
(liter
s), and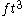 (cubic feet).
(cubic feet).
Volume is one of a pair of conjugate variable
s, the other being pressure. As with all conjugate pairs, the product is a form of energy. The product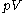 is the energy lost to a system due to mechanical work. This product is one term which makes up enthalpy
is the energy lost to a system due to mechanical work. This product is one term which makes up enthalpy
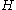 :
: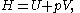
where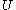 is the internal energy
is the internal energy
of the system.
The second law of thermodynamics
describes constraints on the amount of useful work which can be extracted from a thermodynamic system. In thermodynamic systems where the temperature and volume are held constant, the measure of "useful" work attainable is the Helmholtz free energy
; and in systems where the volume is not held constant, the measure of useful work attainable is the Gibbs free energy
.
Similarly, the appropriate value of heat capacity
to use in a given process depends on whether the process produces a change in volume. The heat capacity is a function of the amount of heat added to a system. In the case of a constant-volume process, all the heat affects the internal energy
of the system (i.e., there is no pV-work, and all the heat affects the temperature). However in a process without a constant volume, the heat addition affects both the internal energy and the work (i.e., the enthalpy); thus the temperature changes by a different amount than in the constant-volume case and a different heat capacity value is required.
 ) is the volume occupied by a unit of mass of a material. In many cases the specific volume is a useful quantity to determine because, as an intensive property, it can be used to determine the complete state of a system in conjunction with another independent intensive variable
) is the volume occupied by a unit of mass of a material. In many cases the specific volume is a useful quantity to determine because, as an intensive property, it can be used to determine the complete state of a system in conjunction with another independent intensive variable
. The specific volume also allows systems to be studied without reference to an exact operating volume, which may not be known (nor significant) at some stages of analysis.
The specific volume of a substance is equal to the reciprocal of its mass density. Specific volume may be expressed in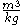 ,
, 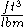 ,
, 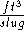 , or
, or 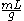 .
.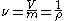
where,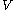 is the volume,
is the volume, 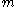 is the mass and
is the mass and 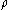 is the density of the material.
is the density of the material.
For an ideal gas
,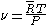
where,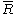 is the specific gas constant,
is the specific gas constant, 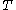 is the temperature and
is the temperature and 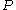 is the pressure of the gas.
is the pressure of the gas.
Specific volume may also refer to molar volume
.
, approximately according to the ideal gas law
:
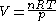
where:
To simplify, a volume of gas may be expressed as the volume it would have in standard conditions for temperature and pressure
, which are 0 °C and 100 kPa.
, to a higher degree depends on vaporization and condensation from or into water, which, in turn, mainly depends on temperature. Therefore, when applying more pressure to a gas saturated with water, all components will initially decrease in volume approximately according to the ideal gas law. However, some of the water will condense until returning to almost the same humidity as before, giving the resulting total volume deviating from what the ideal gas law predicted. Conversely, decreasing temperature would also make some water condense, again making the final volume deviating from predicted by the ideal gas law.
Therefore, gas volume may alternatively be expressed excluding the humidity content: Vd (volume dry). This fraction more accurately follows the ideal gas law. On the contrary Vs (volume saturated) is the volume a gas mixture would have if humidity was added to it until saturation (or 100% relative humidity
).
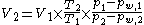
Where, in addition to terms used in the ideal gas law:
For example, calculating how much 1 liter of air (a) at 0°C, 100 kPa, pw = 0 kPa (known as STPD, see below) would fill when breathed into the lungs where it is mixed with water vapor (l), where it quickly becomes 37 °C, 100 kPa, pw = 6.2 kPa (BTPS):
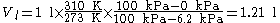
It can be approximated both from partial pressure and molar fraction: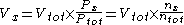
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, the volume of a system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
is an important extensive parameter for describing its thermodynamic state
Thermodynamic state
A thermodynamic state is a set of values of properties of a thermodynamic system that must be specified to reproduce the system. The individual parameters are known as state variables, state parameters or thermodynamic variables. Once a sufficient set of thermodynamic variables have been...
. The specific volume, an intensive property, is the system's volume per unit of mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and temperature
Thermodynamic temperature
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
. For example, volume is related to the pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and temperature
Thermodynamic temperature
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
of an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
by the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
.
The physical volume of a system may or may not coincide with a control volume
Control volume
In fluid mechanics and thermodynamics, a control volume is a mathematical abstraction employed in the process of creating mathematical models of physical processes. In an inertial frame of reference, it is a volume fixed in space or moving with constant velocity through which the fluid flows...
used to analyze the system.
Overview
The volume of a thermodynamic system typically refers to the volume of the working fluid, such as, for example, the fluid within a piston. Changes to this volume may be made through an application of workWork (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
, or may be used to produce work. An isochoric process
Isochoric process
An isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant...
however operates at a constant-volume, thus no work can be produced. Many other thermodynamic processes will result in a change in volume. A polytropic process, in particular, causes changes to the system so that the quantity
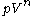 is constant (where
is constant (where 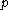 is pressure,
is pressure, 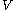 is volume, and
is volume, and  is the polytropic index, a constant). Note that for specific polytropic indexes a polytropic process will be equivalent to a constant-property process. For instance, for very large values of
is the polytropic index, a constant). Note that for specific polytropic indexes a polytropic process will be equivalent to a constant-property process. For instance, for very large values of  approaching infinity, the process becomes constant-volume.
approaching infinity, the process becomes constant-volume.Gases are compressible, thus their volumes (and specific volumes) may be subject to change during thermodynamic processes. Fluids, however, are nearly incompressible, thus their volumes can be often taken as constant. In general, compressibility is defined as the relative volume change of a fluid or solid as a response to a pressure, and may be determined for substances in any phase. Similarly, thermal expansion
Thermal expansion
Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is...
is the tendency of matter to change in volume in response to a change in temperature.
Many thermodynamic cycle
Thermodynamic cycle
A thermodynamic cycle consists of a series of thermodynamic processes transferring heat and work, while varying pressure, temperature, and other state variables, eventually returning a system to its initial state...
s are made up of varying processes, some which maintain a constant volume and some which do not. A vapor-compression refrigeration
Vapor-compression refrigeration
Vapor-compression refrigeration is one of the many refrigeration cycles available for use. It has been and is the most widely used method for air-conditioning of large public buildings, offices, private residences, hotels, hospitals, theaters, restaurants and automobiles...
cycle, for example, follows a sequence where the refrigerant fluid transitions between the liquid and vapor states of matter.
Typical units for volume are
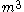 (cubic meters),
(cubic meters), 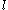 (liter
(literLitér
- External links :*...
s), and
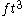 (cubic feet).
(cubic feet).Heat and work
Mechanical work performed on a working fluid causes a change in the mechanical constraints of the system; in other words, for work to occur, the volume must be altered. Hence volume is an important parameter in characterizing many thermodynamic processes where an exchange of energy in the form of work is involved.Volume is one of a pair of conjugate variable
Conjugate variables (thermodynamics)
In thermodynamics, the internal energy of a system is expressed in terms of pairs of conjugate variables such as temperature/entropy or pressure/volume. In fact all thermodynamic potentials are expressed in terms of conjugate pairs....
s, the other being pressure. As with all conjugate pairs, the product is a form of energy. The product
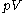 is the energy lost to a system due to mechanical work. This product is one term which makes up enthalpy
is the energy lost to a system due to mechanical work. This product is one term which makes up enthalpyEnthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
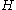 :
: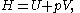
where
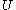 is the internal energy
is the internal energyInternal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
of the system.
The second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
describes constraints on the amount of useful work which can be extracted from a thermodynamic system. In thermodynamic systems where the temperature and volume are held constant, the measure of "useful" work attainable is the Helmholtz free energy
Helmholtz free energy
In thermodynamics, the Helmholtz free energy is a thermodynamic potential that measures the “useful” work obtainable from a closed thermodynamic system at a constant temperature and volume...
; and in systems where the volume is not held constant, the measure of useful work attainable is the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
.
Similarly, the appropriate value of heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
to use in a given process depends on whether the process produces a change in volume. The heat capacity is a function of the amount of heat added to a system. In the case of a constant-volume process, all the heat affects the internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
of the system (i.e., there is no pV-work, and all the heat affects the temperature). However in a process without a constant volume, the heat addition affects both the internal energy and the work (i.e., the enthalpy); thus the temperature changes by a different amount than in the constant-volume case and a different heat capacity value is required.
Specific volume
Specific volume ( ) is the volume occupied by a unit of mass of a material. In many cases the specific volume is a useful quantity to determine because, as an intensive property, it can be used to determine the complete state of a system in conjunction with another independent intensive variable
) is the volume occupied by a unit of mass of a material. In many cases the specific volume is a useful quantity to determine because, as an intensive property, it can be used to determine the complete state of a system in conjunction with another independent intensive variableState postulate
The state postulate is a term used in thermodynamics that defines the given number of properties to a thermodynamic system in a state of equilibrium. The state postulate allows a certain number of properties to be specified to place it in thermodynamic equilibrium...
. The specific volume also allows systems to be studied without reference to an exact operating volume, which may not be known (nor significant) at some stages of analysis.
The specific volume of a substance is equal to the reciprocal of its mass density. Specific volume may be expressed in
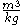 ,
, 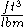 ,
, 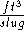 , or
, or 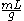 .
.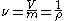
where,
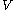 is the volume,
is the volume, 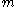 is the mass and
is the mass and 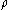 is the density of the material.
is the density of the material.For an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
,
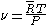
where,
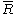 is the specific gas constant,
is the specific gas constant, 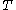 is the temperature and
is the temperature and 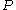 is the pressure of the gas.
is the pressure of the gas.Specific volume may also refer to molar volume
Molar volume
The molar volume, symbol Vm, is the volume occupied by one mole of a substance at a given temperature and pressure. It is equal to the molar mass divided by the mass density...
.
Dependence on pressure and temperature
The volume of gas increases proportionally to absolute temperature and decreases inversely proportionally to pressurePressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, approximately according to the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
:
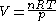
where:
- p is the pressure
- V is the volume
- n is the amount of substanceAmount of substanceAmount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
of gas (moles) - R is the gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
, 8.314 JJouleThe joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
·KKelvinThe kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
−1molMole (unit)The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
−1 - T is the absolute temperature
To simplify, a volume of gas may be expressed as the volume it would have in standard conditions for temperature and pressure
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
, which are 0 °C and 100 kPa.
Humidity exclusion
In contrast to other gas components, water content in air, or humidityHumidity
Humidity is a term for the amount of water vapor in the air, and can refer to any one of several measurements of humidity. Formally, humid air is not "moist air" but a mixture of water vapor and other constituents of air, and humidity is defined in terms of the water content of this mixture,...
, to a higher degree depends on vaporization and condensation from or into water, which, in turn, mainly depends on temperature. Therefore, when applying more pressure to a gas saturated with water, all components will initially decrease in volume approximately according to the ideal gas law. However, some of the water will condense until returning to almost the same humidity as before, giving the resulting total volume deviating from what the ideal gas law predicted. Conversely, decreasing temperature would also make some water condense, again making the final volume deviating from predicted by the ideal gas law.
Therefore, gas volume may alternatively be expressed excluding the humidity content: Vd (volume dry). This fraction more accurately follows the ideal gas law. On the contrary Vs (volume saturated) is the volume a gas mixture would have if humidity was added to it until saturation (or 100% relative humidity
Relative humidity
Relative humidity is a term used to describe the amount of water vapor in a mixture of air and water vapor. It is defined as the partial pressure of water vapor in the air-water mixture, given as a percentage of the saturated vapor pressure under those conditions...
).
General conversion
To compare gas volume between two conditions of different temperature or pressure (1 and 2), assuming nR are the same, the following equation uses humidity exclusion in addition to the ideal gas law: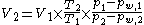
Where, in addition to terms used in the ideal gas law:
- pw is the partial pressure of gaseous water during condition 1 and 2, respectively
For example, calculating how much 1 liter of air (a) at 0°C, 100 kPa, pw = 0 kPa (known as STPD, see below) would fill when breathed into the lungs where it is mixed with water vapor (l), where it quickly becomes 37 °C, 100 kPa, pw = 6.2 kPa (BTPS):
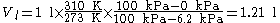
Common conditions
Some common expressions of gas volume with defined or variable temperature, pressure and humidity inclusion are:- ATPS: Ambient temperature (variable) and pressure (variable), saturated (humidity depends on temperature)
- ATPD: Ambient temperature (variable) and pressure (variable), dry (no humidity)
- BTPS: Body Temperature (37 °C or 310 K) and pressure (generally same as ambient), saturated (47 mmHg or 6.2 kPa)
- STPD: Standard temperature (0 °C or 273 K) and pressure (760 mmHg (101.33 kPa) or 100 kPa (750.06 mmHg))Standard conditions for temperature and pressureStandard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
, dry (no humidity)
Conversion factors
| To convert from | To | Multiply by |
|---|---|---|
| ATPS | STPD | [(PA – Pwater S) / PS] * [TS / TA] |
| BTPS | [(PA – Pwater S)/(PA – Pwater B)] * [TB/TA] online calculator | |
| ATPD | (PA – Pwater S)/PA | |
| ATPD | STPD | (PA/PS) * (TS / TA) |
| BTPS | [PA/(PA – Pwater B) * (TB / TA) | |
| ATPS | PA/(PA – Pwater S) | |
| BTPS | http://books.google.com/books?id=1b0iwv8-jGcC&printsec=frontcover#PPA113,M1 | |
| STPD | http://books.google.com/books?id=1b0iwv8-jGcC&printsec=frontcover#PPA113,M1 | |
Legend:
|
||
| Unless else specified in table, then reference is: | ||
Partial volume
The partial volume of a particular gas is the volume which the gas would have if it alone occupied the volume, with unchanged pressure and temperature, and is useful in gas mixtures, e.g. air, to focus on one particular gas component, e.g. oxygen.It can be approximated both from partial pressure and molar fraction:
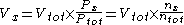
- Vx is the partial volume of any individual gas component (X)
- Vtot is the total volume in gas mixture
- Px is the partial pressurePartial pressureIn a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of gas X - Ptot is the total pressure in gas mixture
- nx is the amount of substanceAmount of substanceAmount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
of a gas (X) - ntot is the total amount of substance in gas mixture

