
Glass electrode
Encyclopedia
A glass electrode is a type of ion-selective electrode
made of a doped glass membrane that is sensitive to a specific ion. It is an important part of the instrumentation for chemical analysis and physico-chemical studies. In modern practice, widely used membranous ion-selective electrodes (ISE, including glasses) are part of a galvanic cell. The electric potential of the electrode system in solution is sensitive to changes in the content of a certain type of ions, which is reflected in the dependence of the electromotive force
(EMF) of galvanic element concentrations of these ions.
s, like H+, Na+, Ag+. The most common glass electrode is the pH
-electrode. Only a few chalcogenide glass
electrodes are sensitive to double-charged ions, like Pb2+, Cd2+ and some others.
There are two main glass-forming systems:
Interference effects are commonly described the semiempirical Nicolsky-Eisenman equation (also known as Nikolsky-Eisenman equation), an extension to the Nernst equation
. It is given by
where E is the emf, E0 the standard electrode potential
, z the ionic valency including the sign, a the activity
, i the ion of interest, j the interfering ions and kij is the selectivity coefficient. The smaller the selectivity coefficient, the less is the interference by j.
To see the interfering effect of Na+ to a pH-electrode:
George Eisenman wrote in his retrospective review:
In 1951 Mikhail Schultz
, first proved rigorously the thermodynamical reversibility of the Na-function of different glasses in different pH ranges (later the functions for other metal ions) that confirmed the validity of one of the key hypotheses of ion-exchange theory, now the Nikolsky-Shultz
-Eisenman thermodynamic ion-exchange theory of GE.
This fact is important because ion-exchange theory was confirmed after a thermodynamically rigorous experimental confirmation of metallic function only. Before, it could be called only as a hypothesis (an epistemological). This opened the way for industrial technology GE, forming ionometry
with them, later with membrane electrodes. In the context of «generalized» theory of glass electrodes, Shultz has created a framework for interpreting a mechanism of the influence of diffusion processes in glasses and resin in their electrode properties, giving new quantitative relationship
, which take into account the dynamic and energetic characteristics of ion exchangers. Schulz introduced a thermodynamic consideration of processes in the membranes. Considering the different abilities of the dissociation of ionogenic groups of the glasses, his theory allows a rigorous analytical way to connect the electrode properties of glasses and ion-exchange resins with their chemical characteristics.....
can be divided into 3 parts:
The effect is usually noticeable at pH > 12, and concentrations of lithium or sodium ions of 0.1 moles per litre or more. Potassium ions usually cause less error than sodium ions.
There are different types of pH glass electrode, some of them have improved characteristics for working in alkaline or acidic media. But almost all electrodes have sufficient properties for working in the most popular pH range from pH = 2 to pH = 12. Special electrodes should be used only for working in aggressive conditions.
Most of text written above is also correct for any ion-exchange electrodes.
The bottom of a pH electrode balloons out into a round thin glass bulb. The pH electrode is best thought of as a tube within a tube. The inside most tube (the inner tube) contains an unchanging 1×10-7 mol/L HCl
solution. Also inside the inner tube is the cathode terminus of the reference probe. The anodic terminus wraps itself around the outside of the inner tube and ends with the same sort of reference probe as was on the inside of the inner tube. It is filled with a reference solution of 0.1 mol/L KCl
and has contact with the solution on the outside of the pH probe by way of a porous plug that serves as a salt bridge.
This device is essentially a galvanic cell
that can be schematically represented as:
In this schematic representation of the galvanic cell, one will note the rigorous symmetry between the left and the right members as seen from the center of the row occupied by the "Test Solution" (the solution whose the pH must be measured). In other words, the glass membrane and the ceramic junction occupies both the same relative place in each respective electrode (indicative (sensing) electrode or reference electrode). The double "pipe symbol" (||) brackets a diffusive barrier avoiding (glass membrane), or slowing down (ceramic junction), the mixing of the different solutions. By using the same electrodes on the left and right, any potential generated
The measuring part of the electrode, the glass bulb on the bottom, is coated both inside and out with a ~10 nm layer of a hydrated gel
. These two layers are separated by a layer of dry glass. The silica glass structure (that is, the conformation of its atomic structure) is shaped in such a way that it allows Na+
ions some mobility. The metal cations (Na+) in the hydrated gel diffuse out of the glass and into solution while H+ from solution can diffuse into the hydrated gel. It is the hydrated gel, which makes the pH electrode an ion-selective electrode.
H+ does not cross through the glass membrane of the pH electrode, it is the Na+ which crosses and allows for a change in free energy
. When an ion diffuses from a region of activity to another region of activity, there is a free energy change and this is what the pH meter actually measures. The hydrated gel membrane is connected by Na+ transport and thus the concentration of H+ on the outside of the membrane is 'relayed' to the inside of the membrane by Na+.
All glass pH electrodes have extremely high electric resistance from 50 to 500 MΩ. Therefore, the glass electrode can be used only with a high input-impedance measuring device like a pH meter
, or, more generically, a high input-impedance voltmeter which is called an electrometer
.
Ion selective electrode
An ion-selective electrode , also known as a specific ion electrode , is a transducer that converts the activity of a specific ion dissolved in a solution into an electrical potential, which can be measured by a voltmeter or pH meter. The voltage is theoretically dependent on the logarithm of the...
made of a doped glass membrane that is sensitive to a specific ion. It is an important part of the instrumentation for chemical analysis and physico-chemical studies. In modern practice, widely used membranous ion-selective electrodes (ISE, including glasses) are part of a galvanic cell. The electric potential of the electrode system in solution is sensitive to changes in the content of a certain type of ions, which is reflected in the dependence of the electromotive force
Electromotive force
In physics, electromotive force, emf , or electromotance refers to voltage generated by a battery or by the magnetic force according to Faraday's Law, which states that a time varying magnetic field will induce an electric current.It is important to note that the electromotive "force" is not a...
(EMF) of galvanic element concentrations of these ions.
History
The first studies of glass electrodes (GE) found different sensitivities of different glasses to change of the medium's acidity (pH), due to effects of the alkali metal ions.- 1906 — M. Cramer determined that the electric potential that arises between parts of the fluid, located on opposite sides of the glass membrane is proportional to the concentration of acid (hydrogen ion concentration).
- 1909 — S. P. L. SørensenS. P. L. SørensenSøren Peder Lauritz Sørensen was a Danish chemist, famous for the introduction of the concept of pH, a scale for measuring acidity and basicity. He was born in Havrebjerg, Denmark....
introduced the concept of pHPHIn chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
. - 1909 — F. HaberFritz HaberFritz Haber was a German chemist, who received the Nobel Prize in Chemistry in 1918 for his development for synthesizing ammonia, important for fertilizers and explosives. Haber, along with Max Born, proposed the Born–Haber cycle as a method for evaluating the lattice energy of an ionic solid...
and Z. KlemensiewiczZygmunt KlemensiewiczZygmunt Aleksander Klemensiewicz was a Polish physicist and physical chemist.Early in his career , he made a pioneering contribution to the development of the glass electrode.- Life and career :In years 1913 - 1914, he worked with Marie Curie in Paris...
publicized on January 28, 1909 results of their research on the glass electrode in The Society of Chemistry in KarlsruheKarlsruheThe City of Karlsruhe is a city in the southwest of Germany, in the state of Baden-Württemberg, located near the French-German border.Karlsruhe was founded in 1715 as Karlsruhe Palace, when Germany was a series of principalities and city states...
(first publication — The Journal of Physical Chemistry by W. OstwaldWolfgang OstwaldCarl Wilhelm Wolfgang Ostwald was a German chemist and biologist researching colloids.Ostwald was born in Riga, the son of the 1909 winner of the Nobel Prize in Chemistry, Wilhelm Ostwald, and died in Dresden.-Works:...
and J. H. van 't HoffJacobus Henricus van 't HoffJacobus Henricus van 't Hoff, Jr. was a Dutch physical and organic chemist and the first winner of the Nobel Prize in chemistry. He is best known for his discoveries in chemical kinetics, chemical equilibrium, osmotic pressure, and stereochemistry...
) — 1909). - 1922 — W. S. Hughes proved that the alkali-silicate GE are similar to hydrogen electrode, reversible with respect to H+.
Applications
Glass electrodes are commonly used for pH measurements. There are also specialized ion sensitive glass electrodes used for determination of concentration of lithium, sodium, ammonium, and other ions. Glass electrodes have been utilized in a wide range of applications — from pure research, control of industrial processes, to analyze foods, cosmetics and comparison of indicators of the environment and environmental regulations: a microelectrode measurements of membrane electrical potential of a biological cell, analysis of soil acidity, etc.Types
Almost all commercial electrodes respond to single charged ionIon
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s, like H
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
-electrode. Only a few chalcogenide glass
Chalcogenide glass
A chalcogenide glass is a glass containing one or more chalcogenide elements. These are Group 16 in the periodic table e.g. sulfur, selenium or tellurium. Such glasses are covalently bonded materials and may be classified as network solids. In effect, the entire glass matrix acts like an...
electrodes are sensitive to double-charged ions, like Pb
There are two main glass-forming systems:
- silicateSilicateA silicate is a compound containing a silicon bearing anion. The great majority of silicates are oxides, but hexafluorosilicate and other anions are also included. This article focuses mainly on the Si-O anions. Silicates comprise the majority of the earth's crust, as well as the other...
matrix based on molecular network of silicon dioxideSilicon dioxideThe chemical compound silicon dioxide, also known as silica , is an oxide of silicon with the chemical formula '. It has been known for its hardness since antiquity...
(SiO2) with additions of other metal oxides, such as Na, K, Li, Al, B, Ca, etc. - chalcogenideChalcogenideA chalcogenide is a chemical compound consisting of at least one chalcogen ion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term is more commonly reserved for sulfides, selenides, and tellurides, rather than...
matrix based on molecular network of AsS, AsSe, AsTe.
Interfering ions
Because of the ion-exchange nature of the glass membrane, it is possible for some other ions to concurrently interact with ion-exchange centers of the glass and to distort the linear dependence of the measured electrode potential on pH or other electrode function. In some cases it is possible to change the electrode function from one ion to another. For example, some silicate pNa electrodes can be changed to pAg function by soaking in a silver salt solution.Interference effects are commonly described the semiempirical Nicolsky-Eisenman equation (also known as Nikolsky-Eisenman equation), an extension to the Nernst equation
Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
. It is given by

where E is the emf, E0 the standard electrode potential
Standard electrode potential
In electrochemistry, the standard electrode potential, abbreviated E° or E , is the measure of individual potential of a reversible electrode at standard state, which is with solutes at an effective concentration of 1 mol dm−3, and gases at a pressure of 1 atm...
, z the ionic valency including the sign, a the activity
Activity
Activity may mean:* Action , in general* The Aristotelian concept of energeia, Latinized as actus* Physical exercise* Activity , a major task in Unified Modeling Language...
, i the ion of interest, j the interfering ions and kij is the selectivity coefficient. The smaller the selectivity coefficient, the less is the interference by j.
To see the interfering effect of Na+ to a pH-electrode:

Metallic function of the glass electrode
Before the 1950s, there was no explanation for some important aspects of the behavior of glass electrodes (GE) and the factual reversibility of this behavior. Some authors have refuted the existence of a particular function at GE in such solutions where they do not behave fully as hydrogen electrode, denying the phenomena of these functions, which were attributed by researchers as an incorrect interpretation of the structural changes in the surface layers of glass; it was mistakenly attributing the changes of EMF element to change in the capacity of GE, and therefore received too large values of pH.George Eisenman wrote in his retrospective review:
In 1951 Mikhail Schultz
Mikhail Shultz
Mikhail Mikhaylovich Shultz , was a Soviet/Russian physical chemist, artist. Proceedings of the thermodynamic theory, the thermodynamics of heterogeneous systems, the theory of glasses, chemistry and electrochemistry of glass, membrane electrochemistry, the theory of ion exchange and phase...
, first proved rigorously the thermodynamical reversibility of the Na-function of different glasses in different pH ranges (later the functions for other metal ions) that confirmed the validity of one of the key hypotheses of ion-exchange theory, now the Nikolsky-Shultz
Mikhail Shultz
Mikhail Mikhaylovich Shultz , was a Soviet/Russian physical chemist, artist. Proceedings of the thermodynamic theory, the thermodynamics of heterogeneous systems, the theory of glasses, chemistry and electrochemistry of glass, membrane electrochemistry, the theory of ion exchange and phase...
-Eisenman thermodynamic ion-exchange theory of GE.
This fact is important because ion-exchange theory was confirmed after a thermodynamically rigorous experimental confirmation of metallic function only. Before, it could be called only as a hypothesis (an epistemological). This opened the way for industrial technology GE, forming ionometry
Ionometer
The term ionometer was originally applied to a device for measuring the intensity of ionising radiation. Examples of radiation detectors described as ionometers can be found through to the 1950s but the term more often now means a device for measuring the chemical ion concentration of a...
with them, later with membrane electrodes. In the context of «generalized» theory of glass electrodes, Shultz has created a framework for interpreting a mechanism of the influence of diffusion processes in glasses and resin in their electrode properties, giving new quantitative relationship
Quantitative structure-activity relationship
Quantitative structure–activity relationship or QSPR is the process by which chemical structure is quantitatively correlated with a well defined process, such as biological activity or chemical reactivity.For example, biological activity can be expressed quantitatively as the concentration of a...
, which take into account the dynamic and energetic characteristics of ion exchangers. Schulz introduced a thermodynamic consideration of processes in the membranes. Considering the different abilities of the dissociation of ionogenic groups of the glasses, his theory allows a rigorous analytical way to connect the electrode properties of glasses and ion-exchange resins with their chemical characteristics.....
Range of a pH glass electrode
The pH range at constant concentrationConcentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
can be divided into 3 parts:
- Complete realization of general electrode function, where dependence of potential on pH has linear behavior and within which such electrode really works as ion-selective electrode for pH.
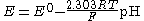
- Alkali error range - at low concentration of hydrogen ionHydrogen ionHydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
s (high values of pH) contributions of interfering alkali metalAlkali metalThe alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
s (like Li, Na, K) are comparable with the one of hydrogen ions. In this situation dependence of the potential on pH become non-linear.
The effect is usually noticeable at pH > 12, and concentrations of lithium or sodium ions of 0.1 moles per litre or more. Potassium ions usually cause less error than sodium ions.
- AcidAcidAn acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
ic error range – at very high concentration of hydrogen ions (low values of pH) the dependence of the electrode on pH becomes non-linear and the influence of the anions in the solution also becomes noticeable. These effects usually become noticeable at pH < -1.
There are different types of pH glass electrode, some of them have improved characteristics for working in alkaline or acidic media. But almost all electrodes have sufficient properties for working in the most popular pH range from pH = 2 to pH = 12. Special electrodes should be used only for working in aggressive conditions.
Most of text written above is also correct for any ion-exchange electrodes.
Construction
A typical modern pH probe is a combination electrode, which combines both the glass and reference electrodes into one body. The combination electrode consists of the following parts (see the drawing):- a sensing part of electrode, a bulb made from a specific glass
- internal electrode, usually silver chloride electrodeSilver chloride electrodeA silver chloride electrode is a type of reference electrode, commonly used in electrochemical measurements. For example, it is usually the internal reference electrode in pH meters...
or calomel electrodeSaturated calomel electrodeThe Saturated calomel electrode is a reference electrode based on the reaction between elemental mercury and mercury chloride. The aqueous phase in contact with the mercury and the mercury chloride is a saturated solution of potassium chloride in water... - internal solution, usually a pH=7 bufferedBuffer solutionA buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
solution of 0.1 mol/L KCl for pH electrodes or 0.1 mol/L MeCl for pMe electrodes - when using the silver chloride electrodeSilver chloride electrodeA silver chloride electrode is a type of reference electrode, commonly used in electrochemical measurements. For example, it is usually the internal reference electrode in pH meters...
, a small amount of AgCl can precipitate inside the glass electrode - reference electrode, usually the same type as 2
- reference internal solution, usually 0.1 mol/L KCl
- junction with studied solution, usually made from ceramicCeramicA ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
s or capillary with asbestosAsbestosAsbestos is a set of six naturally occurring silicate minerals used commercially for their desirable physical properties. They all have in common their eponymous, asbestiform habit: long, thin fibrous crystals...
or quartz fiber. - body of electrode, made from non-conductive glass or plastics.
The bottom of a pH electrode balloons out into a round thin glass bulb. The pH electrode is best thought of as a tube within a tube. The inside most tube (the inner tube) contains an unchanging 1×10-7 mol/L HCl
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
solution. Also inside the inner tube is the cathode terminus of the reference probe. The anodic terminus wraps itself around the outside of the inner tube and ends with the same sort of reference probe as was on the inside of the inner tube. It is filled with a reference solution of 0.1 mol/L KCl
Potassium chloride
The chemical compound potassium chloride is a metal halide salt composed of potassium and chlorine. In its pure state, it is odorless and has a white or colorless vitreous crystal appearance, with a crystal structure that cleaves easily in three directions. Potassium chloride crystals are...
and has contact with the solution on the outside of the pH probe by way of a porous plug that serves as a salt bridge.
Galvanic cell schematic representation
This section describe the functioning of two distinct types of electrodes as one unit which combines both the glass electrode and the reference electrode into one body. It deserves some explanations.This device is essentially a galvanic cell
Galvanic cell
A Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
that can be schematically represented as:
- Glass electrode || Reference Solution || Test Solution || Glass electrode
- Ag(s) | AgCl(s) | KCl(aq) || 1×10-7M H+ solution || glass membrane || Test Solution || ceramic junction || KCl(aq) | AgCl(s) | Ag(s)
In this schematic representation of the galvanic cell, one will note the rigorous symmetry between the left and the right members as seen from the center of the row occupied by the "Test Solution" (the solution whose the pH must be measured). In other words, the glass membrane and the ceramic junction occupies both the same relative place in each respective electrode (indicative (sensing) electrode or reference electrode). The double "pipe symbol" (||) brackets a diffusive barrier avoiding (glass membrane), or slowing down (ceramic junction), the mixing of the different solutions. By using the same electrodes on the left and right, any potential generated
The measuring part of the electrode, the glass bulb on the bottom, is coated both inside and out with a ~10 nm layer of a hydrated gel
Gel
A gel is a solid, jelly-like material that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state...
. These two layers are separated by a layer of dry glass. The silica glass structure (that is, the conformation of its atomic structure) is shaped in such a way that it allows Na+
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
ions some mobility. The metal cations (Na+) in the hydrated gel diffuse out of the glass and into solution while H+ from solution can diffuse into the hydrated gel. It is the hydrated gel, which makes the pH electrode an ion-selective electrode.
H+ does not cross through the glass membrane of the pH electrode, it is the Na+ which crosses and allows for a change in free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
. When an ion diffuses from a region of activity to another region of activity, there is a free energy change and this is what the pH meter actually measures. The hydrated gel membrane is connected by Na+ transport and thus the concentration of H+ on the outside of the membrane is 'relayed' to the inside of the membrane by Na+.
All glass pH electrodes have extremely high electric resistance from 50 to 500 MΩ. Therefore, the glass electrode can be used only with a high input-impedance measuring device like a pH meter
PH meter
A pH meter is an electronic instrument used for measuring the pH of a liquid...
, or, more generically, a high input-impedance voltmeter which is called an electrometer
Electrometer
An electrometer is an electrical instrument for measuring electric charge or electrical potential difference. There are many different types, ranging from historical hand-made mechanical instruments to high-precision electronic devices...
.
Storage
Between measurements any glass and membrane electrodes should be kept in the solution of its own ion (Ex. pH glass electrode should be kept in 0.1 mol/L HCl or 0.1 mol/L H2SO4). It is necessary to prevent the glass membrane from drying out.See also
- Potentiometry
- Ion-selective electrodes
- ISFETISFETISFET pH electrode also redirects here.An ISFET is an ion-sensitive field-effect transistor used for measuring ion concentrations in solution; when the ion concentration changes, the current through the transistor will change accordingly. Here, the solution is used as the gate electrode...
pH electrode - Chalcogenide glassChalcogenide glassA chalcogenide glass is a glass containing one or more chalcogenide elements. These are Group 16 in the periodic table e.g. sulfur, selenium or tellurium. Such glasses are covalently bonded materials and may be classified as network solids. In effect, the entire glass matrix acts like an...
- Quinhydrone electrodeQuinhydrone electrodeThe quinhydrone electrode is a type of redox electrode which can be used to measure the hydrogen ion concentration of a solution in a chemical experiment. It provides an alternative to the commonly used glass electrode in a pH meter....

