Hydrogen economy
Encyclopedia
The hydrogen economy is a proposed system of delivering energy using hydrogen
. The term hydrogen economy was coined by John Bockris
during a talk he gave in 1970 at General Motors
(GM) Technical Center.
Hydrogen advocates promote hydrogen as potential fuel for motive power
(including cars and boats), the energy needs of buildings and portable electronics. Free hydrogen does not occur naturally in quantity, and thus it must be generated from some other energy source by steam reformation of natural gas
or another method. Hydrogen is therefore an energy carrier
(like electricity), not a primary energy source (like coal). The utility of a hydrogen economy depends on issues of energy sourcing
, including fossil fuel
use, climate change
, and sustainable energy
generation.
 A hydrogen economy is proposed to solve some of the negative effects of using hydrocarbon
A hydrogen economy is proposed to solve some of the negative effects of using hydrocarbon
fuels where the carbon is released to the atmosphere. Modern interest in the hydrogen economy can generally be traced to a 1970 technical report by Lawrence W. Jones of the University of Michigan
.
In the current hydrocarbon economy
, transportation is fueled primarily by petroleum
. Burning of hydrocarbon fuels emits carbon dioxide
and other pollutants. The supply of economically usable hydrocarbon resources in the world is limited, and the demand for hydrocarbon fuels is increasing, particularly in China
, India
and other developing countries.
Proponents of a world-scale hydrogen economy argue that hydrogen can be an environmentally cleaner source of energy to end-users, particularly in transportation applications, without release of pollutants (such as particulate matter) or carbon dioxide at the point of end use. A 2004 analysis asserted that "most of the hydrogen supply chain pathways would release significantly less carbon dioxide into the atmosphere than would gasoline used in hybrid electric vehicles" and that significant reductions in carbon dioxide emissions would be possible if carbon capture or carbon sequestration methods were utilized at the site of energy or hydrogen production.
Hydrogen has a high energy density
by weight
. An Otto cycle
internal combustion engine running on hydrogen is said to have a maximum efficiency of about 38%, 8% higher than gasoline internal combustion engine.
The combination of the fuel cell and electric motor is 2-3 times more efficient than an internal combustion engine. However, the high capital costs of fuel cells, about $5,500/kW in 2002, are one of the major obstacles of its development, meaning that the fuel cell is only technically, but not economically, more efficient than an internal combustion engine.
Other technical obstacles include hydrogen storage issues and the purity requirement of hydrogen used in fuel cells – with current technology, an operating fuel cell requires the purity of hydrogen to be as high as 99.999%. On the other hand, hydrogen engine conversion technology is more economical than fuel cells.
 Hydrogen production is a large and growing industry. Globally, some 50 million metric ton
Hydrogen production is a large and growing industry. Globally, some 50 million metric ton
s of hydrogen, equal to about 170 million tons of oil equivalent, were produced in 2004. The growth rate is around 10% per year. Within the United States
, 2004 production was about 11 million metric tons (MMT), an average power flow of 48 gigawatts. (For comparison, the average electric production in 2003 was some 442 gigawatts.) As of 2005, the economic value of all hydrogen produced worldwide is about $135 billion per year.
There are two primary uses for hydrogen today. About half is used to produce ammonia
(NH3) via the Haber process
, which is then used directly or indirectly as fertilizer
. Because both the world population
and the intensive agriculture used to support it are growing, ammonia demand is growing. The other half of current hydrogen production is used to convert heavy petroleum
sources into lighter fractions suitable for use as fuels. This latter process is known as hydrocracking. Hydrocracking represents an even larger growth area, since rising oil prices encourage oil companies to extract poorer source material, such as tar sands and oil shale
. The scale economies inherent in large scale oil refining and fertilizer manufacture make possible on-site production and "captive" use. Smaller quantities of "merchant" hydrogen are manufactured and delivered to end users as well.
If energy for hydrogen production were available (from wind, solar, fission or fusion nuclear power etc.), use of the substance for hydrocarbon synfuel production could expand captive use of hydrogen by a factor of 5 to 10. Present U.S. use of hydrogen for hydrocracking is roughly 4 million metric tons per year (4 MMT/yr). It is estimated that 37.7 MMT/yr of hydrogen would be sufficient to convert enough domestic coal to liquid fuels to end U.S. dependence on foreign oil importation, and less than half this figure to end dependence on Middle East oil. Coal liquefaction
would present significantly worse emissions of carbon dioxide than does the current system of burning fossil petroleum, but it would eliminate the political and economic vulnerabilities inherent in oil importation.
Currently, global hydrogen production is 48% from natural gas
, 30% from oil, and 18% from coal
; water electrolysis
accounts for only 4%. The distribution of production reflects the effects of thermodynamic constraints on economic choices: of the four methods for obtaining hydrogen, partial combustion of natural gas in a NGCC (natural gas combined cycle) power plant offers the most efficient chemical pathway and the greatest off-take of usable heat energy.
The large market and sharply rising prices in fossil fuels have also stimulated great interest in alternate, cheaper means of hydrogen production. As of 2002, most hydrogen is produced on site and the cost is approximately $0.32/lb and, if not produced on site, the cost of liquid hydrogen is about $1.00/lb to $1.40/lb.
. Linking its centralized production to a fleet of light-duty fuel cell vehicle
s would require the siting and construction of a distribution infrastructure with large investment of capital. Further, the technological challenge of providing safe, energy-dense storage of hydrogen on-board the vehicle must be overcome to provide sufficient range between fillups.
or water. The former consumes the fossil resource and produces carbon dioxide, but often requires no further energy input beyond the fossil fuel. Decomposing
water requires electrical or heat input, generated from some primary energy source (fossil fuel
, nuclear power
or a renewable energy
). Note that the energy provided by the energy source provides all of the energy that is available in the hydrogen fuel.
, which uses fossil fuels such as natural gas, oil, or coal. The energy content of the produced hydrogen is less than the energy content of the original fuel, some of it being lost as excessive heat during production. Steam reforming leads to carbon dioxide emissions, in the same way as a car engine would do.
A small part (4% in 2006) is produced by electrolysis
using electricity and water, consuming approximately 50 kilowatt-hours of electricity per kilogram of hydrogen produced.
or Kvaerner carbon black
& hydrogen
process (CB&H) is a method, developed in the 1980s by a Norwegian
company of the same name, for the production of hydrogen from hydrocarbons (CnHm), such as methane
, natural gas
and biogas
.
Of the available energy of the feed, approximately 48% is contained in the Hydrogen, 40% is contained in activated carbon
and 10% in superheated steam.
is the fermentative
conversion of organic substrate to biohydrogen
manifested by a diverse group bacteria
using multi enzyme
systems involving three steps similar to anaerobic conversion
. Dark fermentation
reactions do not require light energy, so they are capable of constantly producing hydrogen
from organic compounds throughout the day and night. Photofermentation
differs from dark fermentation
because it only proceeds in the presence of light
. For example photo-fermentation with Rhodobacter sphaeroides
SH2C can be employed to convert small molecular fatty acids into hydrogen. Electrohydrogenesis
is used in microbial fuel cell
s where hydrogen is produced from organic matter (e.g. from sewage, or solid matter ) while 0.2 - 0.8 V is applied.
Biological hydrogen can be produced in an algae
bioreactor
. In the late 1990s it was discovered that if the algae is deprived of sulfur
it will switch from the production of oxygen
, i.e. normal photosynthesis
, to the production of hydrogen.
Biological hydrogen can be produced in bioreactors that use feedstocks other than algae, the most common feedstock being waste streams. The process involves bacteria feeding on hydrocarbons and excreting hydrogen and CO2. The CO2 can be sequestered successfully by several methods, leaving hydrogen gas. A prototype hydrogen bioreactor using waste as a feedstock is in operation at Welch's grape juice factory in North East, Pennsylvania.
, cordgrass, rice, tomatoes, lupines, algae
 Hydrogen can be made via high pressure electrolysis
Hydrogen can be made via high pressure electrolysis
or low pressure electrolysis of water
. Current best processes have an efficiency of 50% to 80%, so that 1 kg of hydrogen (which has a specific energy
of 143 MJ/kg, about 40 kWh/kg) requires 50 to 79 kWh of electricity. At 8 cents/kWh, that's $4.00/kg, which is with traditional methods 3 to 10 times the price of hydrogen from steam reformation of natural gas. The price difference is due to the efficiency of direct conversion of fossil fuels to produce hydrogen, rather than burning fuel to produce electricity. Hydrogen from natural gas, used to replace e.g. gasoline, emits more CO2 than the gasoline it would replace, and so is no help in reducing greenhouse gases.
is the electrolysis of water
by decomposition of water
(H2O) into oxygen
(O2) and hydrogen
gas (H2) by means of an electric current
being passed through the water. The difference with a standard electrolyzer
is the compressed hydrogen
output around 120-200 Bar
(1740-2900 psi
). By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor
is eliminated, the average energy consumption for internal compression is around 3%.
While nuclear-generated electricity could be used for electrolysis, nuclear heat can be directly applied to split hydrogen from water. High temperature (950–1000 °C) gas cooled nuclear reactors have the potential to split hydrogen from water by thermochemical means using nuclear heat. Research into high-temperature nuclear reactors may eventually lead to a hydrogen supply that is cost-competitive with natural gas steam reforming. General Atomics
predicts that hydrogen produced in a High Temperature Gas Cooled Reactor (HTGR) would cost $1.53/kg. In 2003, steam reforming of natural gas yielded hydrogen at $1.40/kg. At 2005 natural gas prices, hydrogen costs $2.70/kg.
High-temperature electrolysis has been demonstrated in a laboratory, at 108 megajoules (thermal) per kilogram of hydrogen produced, but not at a commercial scale. In addition, this is lower-quality "commercial" grade Hydrogen, unsuitable for use in fuel cells.
(PEC) process which is also named artificial photosynthesis
. Research aimed toward developing higher-efficiency multi-junction cell technology is underway by the photovoltaic industry. If this process is assisted by photocatalysts suspended directly in water instead of using photovoltaic and an electrolytic system, the reaction is in just one step, which can improve efficiency.
is a 100-kilowatt pilot plant at the Plataforma Solar de Almería
in Spain
which uses sunlight to obtain the required 800 to 1,200 °C to heat water. Hydrosol II has been in operation since 2008. The design of this 100-kilowatt pilot plant is based on a modular concept. As a result, it may be possible that this technology could be readily scaled up to the megawatt range by multiplying the available reactor units and by connecting the plant to heliostat
fields (fields of sun-tracking mirrors) of a suitable size.
, around a dozen of these cycles such as the iron oxide cycle
, cerium(IV) oxide-cerium(III) oxide cycle
, zinc zinc-oxide cycle, sulfur-iodine cycle
, copper-chlorine cycle
and hybrid sulfur cycle
are under research and in testing phase to produce hydrogen and oxygen from water and heat without using electricity. These processes can be more efficient than high-temperature electrolysis, typical in the range from 35 % - 49 % LHV efficiency. Thermochemical production of hydrogen using chemical energy from coal or natural gas is generally not considered, because the direct chemical path is more efficient.
None of the thermochemical hydrogen production processes have been demonstrated at production levels, although several have been demonstrated in laboratories.
). Achieving higher pressures necessitates greater use of external energy to power the compression. Alternatively, higher volumetric energy density liquid hydrogen
or slush hydrogen
may be used. However, liquid hydrogen is cryogenic and boils at 20.268 K (–252.882 °C or –423.188 °F). Cryogenic storage cuts weight but requires large liquification
energies. The liquefaction process, involving pressurizing and cooling steps, is energy intensive. The liquefied hydrogen has lower energy density by volume than gasoline by approximately a factor of four, because of the low density of liquid hydrogen — there is actually more hydrogen in a liter of gasoline (116 grams) than there is in a liter of pure liquid hydrogen (71 grams). Liquid hydrogen storage tanks
must also be well insulated to minimize boil off. Ice may form around the tank and help corrode it further if the liquid hydrogen tank insulation fails.
The mass of the tanks needed for compressed hydrogen
reduces the fuel economy of the vehicle. Because it is a small molecule, hydrogen tends to diffuse through any liner material intended to contain it, leading to the embrittlement
, or weakening, of its container.
Distinct from storing molecular hydrogen, hydrogen can be stored as a chemical hydride
or in some other hydrogen-containing compound. Hydrogen gas is reacted with some other materials to produce the hydrogen storage material, which can be transported relatively easily. At the point of use the hydrogen storage material can be made to decompose, yielding hydrogen gas. As well as the mass and volume density problems associated with molecular hydrogen storage, current barriers to practical storage schemes stem from the high pressure and temperature conditions needed for hydride formation and hydrogen release. For many potential systems hydriding and dehydriding kinetics
and heat management are also issues that need to be overcome. A French company McPhy Energy http://www.mcphy.com is developing the first industrial product, based on Magnesium Hydrate, already sold to some major clients such as Iwatani and ENEL.
A third approach is to absorb molecular hydrogen into a solid storage material. Unlike in the hydrides mentioned above, the hydrogen does not dissociate/recombine upon charging/discharging the storage system, and hence does not suffer from the kinetic limitations of many hydride storage systems. Hydrogen densities similar to liquefied hydrogen can be achieved with appropriate absorption media. Some suggested absorbers include MOFs
, nanostructure
d carbons (including CNTs
) and hydrogen clathrate hydrate.
The most common method of on board hydrogen storage in today's demonstration vehicles is as a compressed gas at pressures of roughly 700 bar (70 MPa
).
Underground hydrogen storage
is the practice of hydrogen storage
in underground cave
rns, salt dome
s and depleted oil and gas fields. Large quantities of gaseous hydrogen are stored in underground caverns by ICI
for many years without any difficulties. The storage of large quantities of hydrogen underground can function as grid energy storage
which is essential for the hydrogen economy.
Alternative storage proposal
It has been proposed in a hypothetical renewable energy dominated energy system to use the excess electricity generated by wind, solar photovoltaic, hydro, marine currents and others to make methane (natural gas) by electrolysis of water. The methane could then be injected into the existing gas network to generate electricity and heat on demand to overcome low points of renewable energy production. The process described would be to create hydrogen (which could partly be used directly in fuel cells) and the addition of carbon dioxide CO2 (Sabatier process) to create methane as follows:
CO2 + 4H2 → CH4 + 2H2O
 The hydrogen infrastructure would consist mainly of industrial hydrogen pipeline transport
The hydrogen infrastructure would consist mainly of industrial hydrogen pipeline transport
and hydrogen-equipped filling stations like those found on a hydrogen highway
. Hydrogen stations which were not situated near a hydrogen pipeline would get supply via hydrogen tank
s, compressed hydrogen tube trailers, liquid hydrogen trailer
s, liquid hydrogen tank trucks or dedicated onsite production.
Because of hydrogen embrittlement
of steel, and corrosion natural gas pipes require internal coatings or replacement in order to convey hydrogen. Techniques are well-known; over 700 miles of hydrogen pipeline
currently exist in the United States. Although expensive, pipelines are the cheapest way to move hydrogen. Hydrogen gas piping is routine in large oil-refineries, because hydrogen is used to hydrocrack fuels from crude oil.
Hydrogen piping can in theory be avoided in distributed systems of hydrogen production, where hydrogen is routinely made on site using medium or small-sized generators which would produce enough hydrogen for personal use or perhaps a neighborhood. In the end, a combination of options for hydrogen gas distribution may succeed.
While millions of tons of elemental hydrogen are distributed around the world each year in various ways, bringing hydrogen to individual consumers would require an evolution of the fuel infrastructure. For example, according to GM, 70% of the U.S. population lives near a hydrogen-generating facility but has little public access to that hydrogen. The same study however, shows that building the infrastructure in a systematic way is much more doable and affordable than most people think. For example, one article has noted that hydrogen stations could be put within every 10 miles in metro Los Angeles, and on the highways between LA and neighboring cities like Palm Springs, Las Vegas, San Diego and Santa Barbara, for the cost of a Starbuck's latte for every one of the 15 million residents living in these areas.
One key feature of a hydrogen economy would be that in mobile applications (primarily vehicular transport) energy generation and use could be decoupled. The primary energy source would need no longer travel with the vehicle, as it currently does with hydrocarbon fuels. Instead of tailpipes creating dispersed emissions, the energy (and pollution) could be generated from point sources such as large-scale, centralized facilities with improved efficiency. This would allow the possibility of technologies such as carbon sequestration, which are otherwise impossible for mobile applications. Alternatively, distributed energy generation
schemes (such as small scale renewable energy sources) could be used, possibly associated with hydrogen stations.
Aside from the energy generation, hydrogen production could be centralized, distributed or a mixture of both. While generating hydrogen at centralized primary energy plants promises higher hydrogen production efficiency, difficulties in high-volume, long range hydrogen transportation (due to factors such as hydrogen damage
and the ease of hydrogen diffusion through solid materials) makes electrical energy distribution attractive within a hydrogen economy. In such a scenario, small regional plants or even local filling stations could generate hydrogen using energy provided through the electrical distribution grid. While hydrogen generation efficiency is likely to be lower than for centralized hydrogen generation, losses in hydrogen transport could make such a scheme more efficient in terms of the primary energy used per kilogram of hydrogen delivered to the end user.
The proper balance between hydrogen distribution and long-distance electrical distribution is one of the primary questions that arises about the hydrogen economy.
Again the dilemmas of production sources and transportation of hydrogen can now be overcome using on site (home, business, or fuel station) generation of hydrogen from off grid renewable sources.http://itm-power.com.
Natural gas combined cycle power plants, which account for almost all construction of new electricity generation plants in the United States, generate electricity at efficiencies of 60 percent or greater. Increased demand for electricity, whether due to hydrogen cars or other demand, would have the marginal impact of adding new combined cycle power plants. On this basis, distributed production of hydrogen would be roughly 40% efficient. However, if the marginal impact is referred to today's power grid, with an efficiency of roughly 40% owing to its mix of fuels and conversion methods, the efficiency of distributed hydrogen production would be roughly 25%.
The distributed production of hydrogen in this fashion would be expected to generate air emissions of pollutants and carbon dioxide at various points in the supply chain, e.g., electrolysis, transportation and storage. Such externalities as pollution must be weighed against the potential advantages of a hydrogen economy.
s and turbine
s as the primary way to convert chemical energy into kinetic or electrical energy; hereby eliminating greenhouse gas emissions and pollution from that engine.
Although hydrogen can be used in conventional internal combustion engines, fuel cells,
being electrochemical, have a theoretical efficiency advantage over heat engines. Fuel cells are more expensive to produce than common internal combustion engines, but are becoming cheaper as new technologies and production systems develop.
Some types of fuel cells work with hydrocarbon fuels, while all can be operated on pure hydrogen. In the event that fuel cells become price-competitive with internal combustion engines and turbines, large gas-fired power plants could adopt this technology.
Hydrogen gas must be distinguished as "technical-grade" (five nines pure), which is suitable for applications such as fuel cells, and "commercial-grade", which has carbon- and sulfur-containing impurities, but which can be produced by the much cheaper steam-reformation process. Fuel cells require high purity hydrogen because the impurities would quickly degrade the life of the fuel cell stack.
Much of the interest in the hydrogen economy concept is focused on the use of fuel cells to power electric car
s. Current Hydrogen fuel cells suffer from a low power-to-weight ratio
. Fuel cells are much more efficient than internal combustion engines, and produce no harmful emissions. If a practical method of hydrogen storage
is introduced, and fuel cells become cheaper, they can be economically viable to power hybrid
fuel cell/battery vehicles, or purely fuel cell-driven ones. The economic viability of fuel cell powered vehicles will improve as the hydrocarbon fuels used in internal combustion engines become more expensive, because of the depletion of easily accessible reserves or economic accounting of environmental impact through such measures as carbon tax
es.
Other fuel cell technologies based on the exchange of metal ions (i.e. zinc-air fuel cells
) are typically more efficient at energy conversion than hydrogen fuel cells, but the widespread use of any electrical energy → chemical energy → electrical energy systems would necessitate the production of electricity.
has dropped its plans to develop hydrogen cars, stating that "The next major step in Ford’s plan is to increase over time the volume of electrified vehicles".
An accounting of the energy utilized during a thermodynamic process, known as an energy balance, can be applied to automotive fuels. With today's technology, the manufacture of hydrogen via steam reforming
can be accomplished with a thermal efficiency of 75 to 80 percent. Additional energy will be required to liquefy or compress the hydrogen, and to transport it to the filling station via truck or pipeline. The energy that must be utilized per kilogram to produce, transport and deliver hydrogen (i.e., its well-to-tank energy use) is approximately 50 megajoules using technology available in 2004. Subtracting this energy from the enthalpy of one kilogram of hydrogen, which is 141 megajoules, and dividing by the enthalpy, yields a thermal energy efficiency of roughly 60%. Gasoline, by comparison, requires less energy input, per gallon, at the refinery, and comparatively little energy is required to transport it and store it owing to its high energy density per gallon at ambient temperatures. Well-to-tank, the supply chain for gasoline is roughly 80% efficient (Wang, 2002). The most efficient distribution however is electrical
, which is typically 95% efficient. Electric vehicles
are typically 3 to 4 times as efficient as hydrogen powered vehicles
.
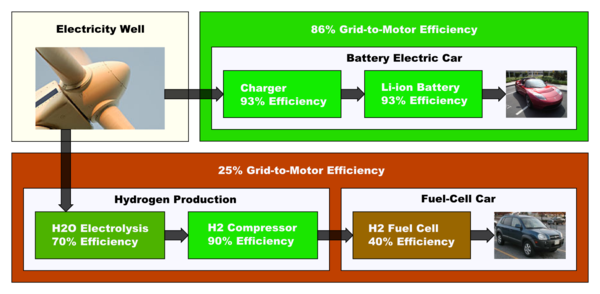 A study of the well-to-wheels efficiency of hydrogen vehicle
A study of the well-to-wheels efficiency of hydrogen vehicle
s compared to other vehicles in the Norwegian energy system indicates that hydrogen fuel-cell vehicles tend to be about a third as efficient as EVs when electrolysis is used, with hydrogen Internal Combustion Engines (ICE) being barely a sixth as efficient. Even in the case where hydrogen fuel cells get their hydrogen from natural gas reformation rather than electrolysis, and EVs get their power from a natural gas power plant, the EVs still come out ahead 35% to 25% (and only 13% for a H2 ICE). This compares to 14% for a gasoline ICE, 27% for a gasoline ICE hybrid, and 17% for a diesel ICE, also on a well-to-wheels basis.
, silane
, and ethylene oxide
. That means that whatever the mix proportion between air and hydrogen, a hydrogen leak will most likely lead to an explosion, not a mere flame, when a flame or spark ignites the mixture. This makes the use of hydrogen particularly dangerous in enclosed areas such as tunnels or underground parking. Pure hydrogen-oxygen flames burn in the ultraviolet
color range and are nearly invisible to the naked eye, so a flame detector
is needed to detect if a hydrogen leak is burning. Hydrogen is odorless and leaks cannot be detected by smell.
Hydrogen codes and standards are code
s and standards for hydrogen fuel cell vehicle
s, stationary fuel cell applications and portable fuel cell applications
. There are codes and standards for the safe handling and storage of hydrogen, for example the Standard for the installation of stationary fuel cell power systems from the National Fire Protection Association
.
Codes and standards have repeatedly been identified as a major institutional barrier to deploying hydrogen technologies
and developing a hydrogen economy. To enable the commercialization of hydrogen in consumer products, new model building codes and equipment and other technical standards are developed and recognized by federal, state, and local governments.
One of the measures on the roadmap is to implement higher safety standards like early leak detection with hydrogen sensors. The Canadian Hydrogen Safety Program concluded that hydrogen fueling is as safe as, or safer than, CNG fueling. The European Commission has funded the first higher educational program in the world in hydrogen safety engineering at the University of Ulster
. It is expected that the general public will be able to use hydrogen technologies in everyday life with at least the same level of safety and comfort as with today's fossil fuels.
, or by fossil fuel reforming. Reforming a fossil fuel leads to a higher emissions of carbon dioxide compared with direct use of the fossil fuel in an internal combustion engine. Similarly, if hydrogen is produced by electrolysis from fossil-fuel powered generators, increased carbon dioxide is emitted in comparison with direct use of the fossil fuel.
Using renewable energy source to generate hydrogen by electrolysis would require greater energy input than direct use of the renewable energy to operate electric vehicles, because of the extra conversion stages and losses in distribution.
Like any internal combustion engine, an ICE running on hydrogen may produce nitrous oxides and other pollutants. Air input into the combustion cylinder is approximately 78% nitrogen, and the N2 molecule has a binding energy
of approximately 226 kilocalories per mole. The hydrogen reaction has sufficient energy to break this bond and produce unwanted components such as nitric acid
(HNO3), and hydrogen cyanide gas (HCN), both toxic byproducts. Nitrogen compound emissions from internal combustion engines are a root cause of smog.
Hydrogen as transportation fuel, however, is mainly used for fuel cells that do not produce greenhouse gas emission, but water.
There have also been some concerns over possible problems related to hydrogen gas leakage. Molecular hydrogen leaks slowly from most containment vessels. It has been hypothesized that if significant amounts of hydrogen gas (H2) escape, hydrogen gas may, because of ultraviolet radiation, form free radicals (H) in the stratosphere. These free radicals would then be able to act as catalysts for ozone depletion
. A large enough increase in stratospheric hydrogen from leaked H2 could exacerbate the depletion process. However, the effect of these leakage problems may not be significant. The amount of hydrogen that leaks today is much lower (by a factor of 10–100) than the estimated 10–20% figure conjectured by some researchers; for example, in Germany
, the leakage rate is only 0.1% (less than the natural gas leak rate of 0.7%). At most, such leakage would likely be no more than 1–2% even with widespread hydrogen use, using present technology.
es, or in battery electric vehicle
s may have a significant economic advantage because there are fewer conversion processes required between primary energy source and point of use.
The barrier to lowering the price of high purity hydrogen is a cost of more than 35 kWh of electricity used to generate each kilogram of hydrogen gas.
Demonstrated advances in electrolyzer and fuel cell technology by ITM Power are claimed to have made significant in-roads into addressing the cost of electrolysing water to make hydrogen. Cost reduction would make hydrogen from off-grid renewable sources economic for refueling vehicles.
Hydrogen pipelines are more expensive than even long-distance electric lines. Hydrogen is about three times bulkier in volume than natural gas for the same enthalpy
. Hydrogen accelerates the cracking of steel (hydrogen embrittlement
), which increases maintenance costs, leakage rates, and material costs. The difference in cost is likely to expand with newer technology: wires suspended in air can use higher voltage with only marginally increased material costs, but higher pressure pipes require proportionally more material.
Setting up a hydrogen economy would require huge investments in the infrastructure to store and distribute hydrogen to vehicles. In contrast, battery electric vehicle
s, which are already publicly available, would not necessitate immediate expansion of the existing infrastructure for electricity transmission and distribution. Power plant capacity that now goes unused at night could be used for recharging electric vehicles. A study conducted by the Pacific Northwest National Laboratory for the US Department of Energy in December 2006 found that the idle off-peak grid capacity in the US would be sufficient to power 84% of all vehicles in the US if they all were immediately replaced with electric vehicles.
Different production methods each have differing associated investment and marginal costs. The energy and feedstock could originate from a multitude of sources i.e. natural gas, nuclear, solar, wind, biomass, coal, other fossil fuels, and geothermal.
Natural Gas at Small Scale: Uses steam reformation. Requires 15.9 Mcuft of gas, which, if produced by small 500 kg/day reformers at the point of dispensing (i.e., the filling station), would equate to 777,000 reformers costing $1 trillion dollars and producing 150 million tons of hydrogen gas annually. Obviates the need for distribution infrastructure dedicated to hydrogen. $3.00 per GGE (Gallons of Gasoline Equivalent)
Nuclear: Provides energy for electrolysis of water. Would require 240,000 tons of unenriched uranium — that's 2,000 600-megawatt power plants, which would cost $840 billion, or about $2.50 per GGE.
Solar: Provides energy for electrolysis of water. Would require 2,500 kWh of sun per square meter, 113 million 40-kilowatt systems, which would cost $22 trillion, or about $9.50 per GGE.
Wind: Provides energy for electrolysis of water. At 7 meters per second average wind speed, it would require 1 million 2-MW wind turbines, which would cost $3 trillion dollars, or about $3.00 per GGE.
Biomass: Gasification plants would produce gas with steam reformation. 1.5 billion tons of dry biomass, 3,300 plants which would require 113.4 million acres (460,000 km²) of farm to produce the biomass. $565 billion dollars in cost, or about $1.90 per GGE
Coal: FutureGen plants use coal gasification then steam reformation. Requires 1 billion tons of coal or about 1,000 275-megawatt plants with a cost of about $500 billion, or about $1 per GGE.
automobile
manufactures have committed to develop vehicles using hydrogen. The distribution of hydrogen for the purpose of transportation is currently being tested around the world, particularly in Portugal
, Iceland
, Norway
, Denmark
, Germany
, California
, Japan and Canada
, but the cost is very high.
Some hospitals have installed combined electrolyzer-storage-fuel cell units for local emergency power. These are advantageous for emergency use because of their low maintenance requirement and ease of location compared to internal combustion driven generators.
Iceland
has committed to becoming the world's first hydrogen economy by the year 2050. Iceland is in a unique position. Presently, it imports all the petroleum products necessary to power its automobiles and fishing fleet
. Iceland has large geothermal resources, so much that the local price of electricity actually is lower than the price of the hydrocarbons that could be used to produce that electricity.
Iceland already converts its surplus electricity into exportable goods and hydrocarbon replacements. In 2002, it produced 2,000 tons of hydrogen gas by electrolysis—primarily for the production of ammonia (NH3) for fertilizer. Ammonia is produced, transported, and used throughout the world, and 90% of the cost of ammonia is the cost of the energy to produce it. Iceland is also developing an aluminium -smelting industry. Aluminium costs are primarily driven by the cost of the electricity to run the smelters. Either of these industries could effectively export all of Iceland's potential geothermal electricity
.
Neither industry directly replaces hydrocarbons. Reykjavík
, Iceland, had a small pilot fleet of city buses running on compressed hydrogen, and research on powering the nation's fishing fleet with hydrogen is under way. For more practical purposes, Iceland might process imported oil with hydrogen to extend it, rather than to replace it altogether.
The Reykjavík buses are part of a larger program, HyFLEET:CUTE, operating hydrogen fueled buses in eight European cities. HyFLEET:CUTE buses also operate in Beijing and Perth (see below).
A pilot project demonstrating a hydrogen economy is operational on the Norwegian
island of Utsira
. The installation combines wind power
and hydrogen power. In periods when there is surplus wind energy, the excess power is used for generating hydrogen by electrolysis
. The hydrogen is stored, and is available for power generation in periods when there is little wind.
A joint venture between NREL and Xcel Energy
is combining wind power
and hydrogen power in the same way in Colorado.
In the Netherlands, one fossil fuel power plant (with full carbon capture) is already scheduled to incorporate the generating of hydrogen. The project is called CGEN and the power plant wil be built in the port of Rotterdam.
Hydro
in Newfoundland and Labrador
are converting the current wind-diesel Power System on the remote island of Ramea into a Wind-Hydrogen Hybrid Power Systems facility.
A similar pilot project on Stuart Island
uses solar power
, instead of wind power
, to generate electricity. When excess electricity is available after the batteries are full, hydrogen is generated by electrolysis and stored for later production of electricity by fuel cell.
The UK
started a fuel cell pilot program in January 2004, the program ran two Fuel cell buses on route 25 in London
until December 2005, and switched to route RV1 until January 2007.
The Hydrogen Expedition is currently working to create a hydrogen fuel cell-powered ship and using it to circumnavigate the globe, as a way to demonstrate the capability of hydrogen fuel cells.
Western Australia's Department of Planning and Infrastructure currently operates three Daimler Chrysler Citaro fuel cell buses as part of its Sustainable Transport Energy for Perth Fuel Cells Bus Trial in Perth. The buses are operated by Path Transit on regular Transperth public bus routes. The trial began in September 2004 and concluded in September 2006. The buses' fuel cells use a proton exchange membrane system and are supplied with raw hydrogen from a BP refinery in Kwinana, south of Perth. The hydrogen is a byproduct of the refinery's industrial process. The buses are refueled at a station in the northern Perth suburb of Malaga.
The United Nations Industrial Development Organization (UNIDO) and the Turkish Ministry of Energy and Natural Resources
have signed in 2003 a $40M Trust Fund Agreement for the creation in Istanbul of the International Centre for Hydrogen Energy Technologies
(UNIDO-ICHET), which started operation in 2004. A hydrogen forklift, a hydrogen cart and a mobile house powered by renewable energies are being demonstrated in UNIDO-ICHET's premises. An uninterruptible power supply system has been working since April 2009 in the headquarters of Istanbul Sea Buses
company.
Hydrogen is simply a method to store and transmit energy. Various alternative energy transmission and storage scenarios which begin with hydrogen production, but do not use it for all parts of the store and transmission infrastructure, may be more economic, in both near and far term. These include:
as an energy carrier is to bond it with nitrogen
from the air to produce ammonia
, which can be easily liquefied, transported, and used (directly or indirectly) as a clean and renewable fuel.
, in non-polluting form, available to automobiles. However, a theoretical alternative to address the same problem is to produced hydrogen centrally and immediately use it to make liquid fuels from a CO2 source. This would eliminate the requirement to transport and store the hydrogen. The source could be CO2 that is produced by fuel-burning power plants. In order to be greenhouse-neutral, the source for CO2 in such a plan would need to be from air, biomass, or other source of CO2 which is already in, or to be released into, the air.). Direct methanol fuel cells are in commercial use, though they are not presently efficient.
, or PHEVs, which use a hybrid strategy of electrical and fuel storage for their energy needs.
Hydrogen storage has been proposed by some to be optimal in a narrow range of energy storage time, probably somewhere between a few days and a few weeks. This range is subject to further narrowing with any improvements in battery technology. It is always possible that some kind of breakthrough in hydrogen storage or generation could occur, but this is unlikely given the physical and chemical limitations of the technical choices are fairly well understood.
has been proposed (note that this is the reverse of the present method of acquiring hydrogen from natural methane, but one that does not require ultimate burning and release of fossil fuel carbon). Captive hydrogen (and carbon dioxide) may be used onsite to synthesize methane, using the Sabatier reaction
. This process is about 80% efficient, reducing the round trip efficiency to about 20 to 30%, depending on the method of fuel utilization. This is even lower than hydrogen, but the storage costs drop by at least a factor of 3, because of methane's higher boiling point and higher energy density. Liquid methane has 3.2 times the energy density of liquid hydrogen and is easier to store. Additionally, the pipe infrastructure (natural gas
pipelines) are already in place. Natural-gas-powered vehicles already exist, and are known to be easier to adapt from existing internal engine technology, than internal combustion autos running directly on hydrogen. Experience with natural gas powered vehicles shows that methane storage is inexpensive, once one has accepted the cost of conversion to store the fuel. However, the cost of alcohol storage is even lower, so this technology would need to produce methane at a considerable savings with regard to alcohol production. Ultimate mature prices of fuels in the competing technologies are not presently known, but both are expected to offer substantial infrastructural savings over attempts to transport and use hydrogen directly.
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
. The term hydrogen economy was coined by John Bockris
John Bockris
John O'Mara Bockris is a former professor of Chemistry at Texas A&M University whose unorthodox views have provoked controversy. He has authored, coauthored or edited more than 700 papers and 22 books principally in electrochemistry but also in environmental chemistry, photoelectrochemistry and...
during a talk he gave in 1970 at General Motors
General Motors
General Motors Company , commonly known as GM, formerly incorporated as General Motors Corporation, is an American multinational automotive corporation headquartered in Detroit, Michigan and the world's second-largest automaker in 2010...
(GM) Technical Center.
Hydrogen advocates promote hydrogen as potential fuel for motive power
Motive power
In thermodynamics, motive power is an agency, as water or steam, used to impart motion. Generally, motive power is defined as a natural agent, as water, steam, wind, electricity, etc., used to impart motion to machinery; a motor; a mover. The term may also define something, as a locomotive or a...
(including cars and boats), the energy needs of buildings and portable electronics. Free hydrogen does not occur naturally in quantity, and thus it must be generated from some other energy source by steam reformation of natural gas
Steam reforming
Fossil fuel reforming is a method of producing hydrogen or other useful products from fossil fuels such as natural gas. This is achieved in a processing device called a reformer which reacts steam at high temperature with the fossil fuel. The steam methane reformer is widely used in industry to...
or another method. Hydrogen is therefore an energy carrier
Energy carrier
According to ISO 13600, an energy carrier is either a substance or a phenomenon that can be used to produce mechanical work or heat or to operate chemical or physical processes....
(like electricity), not a primary energy source (like coal). The utility of a hydrogen economy depends on issues of energy sourcing
Energy development
Energy development is the effort to provide sufficient primary energy sources and secondary energy forms for supply, cost, impact on air pollution and water pollution, mitigation of climate change with renewable energy....
, including fossil fuel
Fossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
use, climate change
Climate change
Climate change is a significant and lasting change in the statistical distribution of weather patterns over periods ranging from decades to millions of years. It may be a change in average weather conditions or the distribution of events around that average...
, and sustainable energy
Sustainable energy
Sustainable energy is the provision of energy that meets the needs of the present without compromising the ability of future generations to meet their needs. Sustainable energy sources include all renewable energy sources, such as hydroelectricity, solar energy, wind energy, wave power, geothermal...
generation.
Rationale

Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
fuels where the carbon is released to the atmosphere. Modern interest in the hydrogen economy can generally be traced to a 1970 technical report by Lawrence W. Jones of the University of Michigan
University of Michigan
The University of Michigan is a public research university located in Ann Arbor, Michigan in the United States. It is the state's oldest university and the flagship campus of the University of Michigan...
.
In the current hydrocarbon economy
Hydrocarbon economy
Hydrocarbon economy is a term stressing that in the current world economy the energy used mostly comes from three hydrocarbons: petroleum, coal, and natural gas. Hydrocarbon economy is often used when talking about possible alternatives like the hydrogen economy....
, transportation is fueled primarily by petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
. Burning of hydrocarbon fuels emits carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and other pollutants. The supply of economically usable hydrocarbon resources in the world is limited, and the demand for hydrocarbon fuels is increasing, particularly in China
People's Republic of China
China , officially the People's Republic of China , is the most populous country in the world, with over 1.3 billion citizens. Located in East Asia, the country covers approximately 9.6 million square kilometres...
, India
India
India , officially the Republic of India , is a country in South Asia. It is the seventh-largest country by geographical area, the second-most populous country with over 1.2 billion people, and the most populous democracy in the world...
and other developing countries.
Proponents of a world-scale hydrogen economy argue that hydrogen can be an environmentally cleaner source of energy to end-users, particularly in transportation applications, without release of pollutants (such as particulate matter) or carbon dioxide at the point of end use. A 2004 analysis asserted that "most of the hydrogen supply chain pathways would release significantly less carbon dioxide into the atmosphere than would gasoline used in hybrid electric vehicles" and that significant reductions in carbon dioxide emissions would be possible if carbon capture or carbon sequestration methods were utilized at the site of energy or hydrogen production.
Hydrogen has a high energy density
Energy density
Energy density is a term used for the amount of energy stored in a given system or region of space per unit volume. Often only the useful or extractable energy is quantified, which is to say that chemically inaccessible energy such as rest mass energy is ignored...
by weight
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
. An Otto cycle
Otto cycle
An Otto cycle is an idealized thermodynamic cycle which describes the functioning of a typical reciprocating piston engine, the thermodynamic cycle most commonly found in automobile engines....
internal combustion engine running on hydrogen is said to have a maximum efficiency of about 38%, 8% higher than gasoline internal combustion engine.
The combination of the fuel cell and electric motor is 2-3 times more efficient than an internal combustion engine. However, the high capital costs of fuel cells, about $5,500/kW in 2002, are one of the major obstacles of its development, meaning that the fuel cell is only technically, but not economically, more efficient than an internal combustion engine.
Other technical obstacles include hydrogen storage issues and the purity requirement of hydrogen used in fuel cells – with current technology, an operating fuel cell requires the purity of hydrogen to be as high as 99.999%. On the other hand, hydrogen engine conversion technology is more economical than fuel cells.
Perspective: current hydrogen market (current hydrogen economy)

Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s of hydrogen, equal to about 170 million tons of oil equivalent, were produced in 2004. The growth rate is around 10% per year. Within the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
, 2004 production was about 11 million metric tons (MMT), an average power flow of 48 gigawatts. (For comparison, the average electric production in 2003 was some 442 gigawatts.) As of 2005, the economic value of all hydrogen produced worldwide is about $135 billion per year.
There are two primary uses for hydrogen today. About half is used to produce ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
(NH3) via the Haber process
Haber process
The Haber process, also called the Haber–Bosch process, is the nitrogen fixation reaction of nitrogen gas and hydrogen gas, over an enriched iron or ruthenium catalyst, which is used to industrially produce ammonia....
, which is then used directly or indirectly as fertilizer
Fertilizer
Fertilizer is any organic or inorganic material of natural or synthetic origin that is added to a soil to supply one or more plant nutrients essential to the growth of plants. A recent assessment found that about 40 to 60% of crop yields are attributable to commercial fertilizer use...
. Because both the world population
World population
The world population is the total number of living humans on the planet Earth. As of today, it is estimated to be billion by the United States Census Bureau...
and the intensive agriculture used to support it are growing, ammonia demand is growing. The other half of current hydrogen production is used to convert heavy petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
sources into lighter fractions suitable for use as fuels. This latter process is known as hydrocracking. Hydrocracking represents an even larger growth area, since rising oil prices encourage oil companies to extract poorer source material, such as tar sands and oil shale
Oil shale
Oil shale, an organic-rich fine-grained sedimentary rock, contains significant amounts of kerogen from which liquid hydrocarbons called shale oil can be produced...
. The scale economies inherent in large scale oil refining and fertilizer manufacture make possible on-site production and "captive" use. Smaller quantities of "merchant" hydrogen are manufactured and delivered to end users as well.
If energy for hydrogen production were available (from wind, solar, fission or fusion nuclear power etc.), use of the substance for hydrocarbon synfuel production could expand captive use of hydrogen by a factor of 5 to 10. Present U.S. use of hydrogen for hydrocracking is roughly 4 million metric tons per year (4 MMT/yr). It is estimated that 37.7 MMT/yr of hydrogen would be sufficient to convert enough domestic coal to liquid fuels to end U.S. dependence on foreign oil importation, and less than half this figure to end dependence on Middle East oil. Coal liquefaction
Coal liquefaction
-Methods:The liquefaction processes are classified as direct conversion to liquids processes and indirect conversion to liquids processeses. Direct processes are carbonization and hydrogenation.-Pyrolysis and carbonization processes:...
would present significantly worse emissions of carbon dioxide than does the current system of burning fossil petroleum, but it would eliminate the political and economic vulnerabilities inherent in oil importation.
Currently, global hydrogen production is 48% from natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
, 30% from oil, and 18% from coal
Coal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
; water electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
accounts for only 4%. The distribution of production reflects the effects of thermodynamic constraints on economic choices: of the four methods for obtaining hydrogen, partial combustion of natural gas in a NGCC (natural gas combined cycle) power plant offers the most efficient chemical pathway and the greatest off-take of usable heat energy.
The large market and sharply rising prices in fossil fuels have also stimulated great interest in alternate, cheaper means of hydrogen production. As of 2002, most hydrogen is produced on site and the cost is approximately $0.32/lb and, if not produced on site, the cost of liquid hydrogen is about $1.00/lb to $1.40/lb.
Production, storage, infrastructure
Today hydrogen is mainly produced (90%) from fossil sourcesFossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
. Linking its centralized production to a fleet of light-duty fuel cell vehicle
Fuel cell vehicle
A Fuel cell vehicle or Fuel Cell Electric Vehicle is a type of hydrogen vehicle which uses a fuel cell to produce electricity, powering its on-board electric motor...
s would require the siting and construction of a distribution infrastructure with large investment of capital. Further, the technological challenge of providing safe, energy-dense storage of hydrogen on-board the vehicle must be overcome to provide sufficient range between fillups.
Methods of production
Molecular hydrogen is not available on Earth in convenient natural reservoirs. Most hydrogen on Earth is bonded to oxygen in water. Manufacturing elemental hydrogen does require the consumption of a hydrogen carrier such as a fossil fuelFossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
or water. The former consumes the fossil resource and produces carbon dioxide, but often requires no further energy input beyond the fossil fuel. Decomposing
Chemical decomposition
Chemical decomposition, analysis or breakdown is the separation of a chemical compound into elements or simpler compounds. It is sometimes defined as the exact opposite of a chemical synthesis. Chemical decomposition is often an undesired chemical reaction...
water requires electrical or heat input, generated from some primary energy source (fossil fuel
Fossil fuel
Fossil fuels are fuels formed by natural processes such as anaerobic decomposition of buried dead organisms. The age of the organisms and their resulting fossil fuels is typically millions of years, and sometimes exceeds 650 million years...
, nuclear power
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
or a renewable energy
Renewable energy
Renewable energy is energy which comes from natural resources such as sunlight, wind, rain, tides, and geothermal heat, which are renewable . About 16% of global final energy consumption comes from renewables, with 10% coming from traditional biomass, which is mainly used for heating, and 3.4% from...
). Note that the energy provided by the energy source provides all of the energy that is available in the hydrogen fuel.
Current production methods
Hydrogen is industrially produced from steam reformingSteam reforming
Fossil fuel reforming is a method of producing hydrogen or other useful products from fossil fuels such as natural gas. This is achieved in a processing device called a reformer which reacts steam at high temperature with the fossil fuel. The steam methane reformer is widely used in industry to...
, which uses fossil fuels such as natural gas, oil, or coal. The energy content of the produced hydrogen is less than the energy content of the original fuel, some of it being lost as excessive heat during production. Steam reforming leads to carbon dioxide emissions, in the same way as a car engine would do.
A small part (4% in 2006) is produced by electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
using electricity and water, consuming approximately 50 kilowatt-hours of electricity per kilogram of hydrogen produced.
Kværner-process
The Kværner-processKværner-process
The Kværner-process or Kvaerner carbon black & hydrogen process is a method, developed in the 1980s by Aker Solutions of Norway, for the production of hydrogen from hydrocarbons , such as methane, natural gas and biogas.- Description :...
or Kvaerner carbon black
Carbon black
Carbon black is a material produced by the incomplete combustion of heavy petroleum products such as FCC tar, coal tar, ethylene cracking tar, and a small amount from vegetable oil. Carbon black is a form of amorphous carbon that has a high surface-area-to-volume ratio, although its...
& hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
process (CB&H) is a method, developed in the 1980s by a Norwegian
Norway
Norway , officially the Kingdom of Norway, is a Nordic unitary constitutional monarchy whose territory comprises the western portion of the Scandinavian Peninsula, Jan Mayen, and the Arctic archipelago of Svalbard and Bouvet Island. Norway has a total area of and a population of about 4.9 million...
company of the same name, for the production of hydrogen from hydrocarbons (CnHm), such as methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
and biogas
Biogas
Biogas typically refers to a gas produced by the biological breakdown of organic matter in the absence of oxygen. Organic waste such as dead plant and animal material, animal dung, and kitchen waste can be converted into a gaseous fuel called biogas...
.
Of the available energy of the feed, approximately 48% is contained in the Hydrogen, 40% is contained in activated carbon
Activated carbon
Activated carbon, also called activated charcoal, activated coal or carbo activatus, is a form of carbon that has been processed to make it extremely porous and thus to have a very large surface area available for adsorption or chemical reactions.The word activated in the name is sometimes replaced...
and 10% in superheated steam.
Biological production
Fermentative hydrogen productionFermentative hydrogen production
Fermentative hydrogen production is the fermentative conversion of organic substrate to biohydrogen manifested by a diverse group of bacteria using multi enzyme systems involving three steps similar to anaerobic conversion. Dark fermentation reactions do not require light energy, so they are...
is the fermentative
Fermentation (biochemistry)
Fermentation is the process of extracting energy from the oxidation of organic compounds, such as carbohydrates, using an endogenous electron acceptor, which is usually an organic compound. In contrast, respiration is where electrons are donated to an exogenous electron acceptor, such as oxygen,...
conversion of organic substrate to biohydrogen
Biohydrogen
Biohydrogen is defined as hydrogen produced biologically, most commonly by algae and bacteria. Biohydrogen is a potential biofuel obtainable from both cultivation and waste organic materials.-Introduction:...
manifested by a diverse group bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
using multi enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
systems involving three steps similar to anaerobic conversion
Anaerobic digestion
Anaerobic digestion is a series of processes in which microorganisms break down biodegradable material in the absence of oxygen. It is used for industrial or domestic purposes to manage waste and/or to release energy....
. Dark fermentation
Dark fermentation
Dark fermentation is the fermentative conversion of organic substrate to biohydrogen. It is a complex process manifested by diverse group of bacteria by a series of biochemical reactions involving three steps similar to anaerobic conversion...
reactions do not require light energy, so they are capable of constantly producing hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
from organic compounds throughout the day and night. Photofermentation
Photofermentation
Photofermentation is the fermentative conversion of organic substrate to biohydrogen manifested by a diverse group of photosynthetic bacteria by a series of biochemical reactions involving three steps similar to anaerobic conversion...
differs from dark fermentation
Dark fermentation
Dark fermentation is the fermentative conversion of organic substrate to biohydrogen. It is a complex process manifested by diverse group of bacteria by a series of biochemical reactions involving three steps similar to anaerobic conversion...
because it only proceeds in the presence of light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
. For example photo-fermentation with Rhodobacter sphaeroides
Rhodobacter sphaeroides
Rhodobacter sphaeroides is a kind of purple bacteria; a group of bacteria that can obtain energy through photosynthesis. Its best growth conditions are anaerobic phototrophy and aerobic chemoheterotrophy in the absence of light. R. sphaeroides is also able to fix nitrogen...
SH2C can be employed to convert small molecular fatty acids into hydrogen. Electrohydrogenesis
Electrohydrogenesis
Electrohydrogenesis or biocatalyzed electrolysis is the name given to a process for generating hydrogen gas from organic matter being decomposed by bacteria. This process uses a modified fuel cell to contain the organic matter and water...
is used in microbial fuel cell
Microbial fuel cell
A microbial fuel cell or biological fuel cell is a bio-electrochemical system that drives a current by mimicking bacterial interactions found in nature....
s where hydrogen is produced from organic matter (e.g. from sewage, or solid matter ) while 0.2 - 0.8 V is applied.
Biological hydrogen can be produced in an algae
Algae
Algae are a large and diverse group of simple, typically autotrophic organisms, ranging from unicellular to multicellular forms, such as the giant kelps that grow to 65 meters in length. They are photosynthetic like plants, and "simple" because their tissues are not organized into the many...
bioreactor
Bioreactor
A bioreactor may refer to any manufactured or engineered device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This...
. In the late 1990s it was discovered that if the algae is deprived of sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
it will switch from the production of oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, i.e. normal photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
, to the production of hydrogen.
Biological hydrogen can be produced in bioreactors that use feedstocks other than algae, the most common feedstock being waste streams. The process involves bacteria feeding on hydrocarbons and excreting hydrogen and CO2. The CO2 can be sequestered successfully by several methods, leaving hydrogen gas. A prototype hydrogen bioreactor using waste as a feedstock is in operation at Welch's grape juice factory in North East, Pennsylvania.
Biocatalysed electrolysis
Besides regular electrolysis, electrolysis using microbes is another possibility. With biocatalysed electrolysis, hydrogen is generated after running through the microbial fuel cell and a variety of aquatic plants can be used. These include reed sweetgrassGlyceria maxima
Glyceria maxima Holmb. is a species of rhizomatous perennial grasses in the mannagrass genus native to Europe and Western Siberia and growing in wet areas such as riverbanks and ponds...
, cordgrass, rice, tomatoes, lupines, algae
Electrolysis of water

High pressure electrolysis
High-pressure electrolysis is the electrolysis of water by decomposition of water into oxygen and hydrogen gas due to the passing of an electric current through the water. The difference with a standard proton exchange membrane electrolyzer is the compressed hydrogen output around 120–200 Bar ...
or low pressure electrolysis of water
Electrolysis of water
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
. Current best processes have an efficiency of 50% to 80%, so that 1 kg of hydrogen (which has a specific energy
Specific energy
Specific energy is defined as the energy per unit mass. Common metric units are J/kg. It is an intensive property. Contrast this with energy, which is an extensive property. There are two main types of specific energy: potential energy and specific kinetic energy. Others are the gray and sievert,...
of 143 MJ/kg, about 40 kWh/kg) requires 50 to 79 kWh of electricity. At 8 cents/kWh, that's $4.00/kg, which is with traditional methods 3 to 10 times the price of hydrogen from steam reformation of natural gas. The price difference is due to the efficiency of direct conversion of fossil fuels to produce hydrogen, rather than burning fuel to produce electricity. Hydrogen from natural gas, used to replace e.g. gasoline, emits more CO2 than the gasoline it would replace, and so is no help in reducing greenhouse gases.
High-pressure electrolysis
High pressure electrolysisHigh pressure electrolysis
High-pressure electrolysis is the electrolysis of water by decomposition of water into oxygen and hydrogen gas due to the passing of an electric current through the water. The difference with a standard proton exchange membrane electrolyzer is the compressed hydrogen output around 120–200 Bar ...
is the electrolysis of water
Electrolysis of water
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
by decomposition of water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
(H2O) into oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(O2) and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
gas (H2) by means of an electric current
Electricity
Electricity is a general term encompassing a variety of phenomena resulting from the presence and flow of electric charge. These include many easily recognizable phenomena, such as lightning, static electricity, and the flow of electrical current in an electrical wire...
being passed through the water. The difference with a standard electrolyzer
Electrolysis of water
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
is the compressed hydrogen
Compressed hydrogen
Compressed hydrogen is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar and 700 bar is used for mobile hydrogen storage in hydrogen vehicles...
output around 120-200 Bar
Bar (unit)
The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar...
(1740-2900 psi
Pounds per square inch
The pound per square inch or, more accurately, pound-force per square inch is a unit of pressure or of stress based on avoirdupois units...
). By pressurising the hydrogen in the electrolyser the need for an external hydrogen compressor
Hydrogen compressor
A hydrogen compressor is a device that increases the pressure of hydrogen by reducing its volume. Compression of hydrogen gas naturally increases its temperature, due to Charles' Law....
is eliminated, the average energy consumption for internal compression is around 3%.
High-temperature electrolysis
Hydrogen can be generated from energy supplied in the form of heat and electricity through high-temperature electrolysis (HTE). Because some of the energy in HTE is supplied in the form of heat, less of the energy must be converted twice (from heat to electricity, and then to chemical form), and so potentially far less energy is required per kilogram of hydrogen produced.While nuclear-generated electricity could be used for electrolysis, nuclear heat can be directly applied to split hydrogen from water. High temperature (950–1000 °C) gas cooled nuclear reactors have the potential to split hydrogen from water by thermochemical means using nuclear heat. Research into high-temperature nuclear reactors may eventually lead to a hydrogen supply that is cost-competitive with natural gas steam reforming. General Atomics
General Atomics
General Atomics is a nuclear physics and defense contractor headquartered in San Diego, California. General Atomics’ research into fission and fusion matured into competencies in related technologies, allowing the company to expand into other fields of research...
predicts that hydrogen produced in a High Temperature Gas Cooled Reactor (HTGR) would cost $1.53/kg. In 2003, steam reforming of natural gas yielded hydrogen at $1.40/kg. At 2005 natural gas prices, hydrogen costs $2.70/kg.
High-temperature electrolysis has been demonstrated in a laboratory, at 108 megajoules (thermal) per kilogram of hydrogen produced, but not at a commercial scale. In addition, this is lower-quality "commercial" grade Hydrogen, unsuitable for use in fuel cells.
Photoelectrochemical water splitting
Using electricity produced by photovoltaic systems offers the cleanest way to produce hydrogen. Water is broken into hydrogen and oxygen by electrolysis—a photoelectrochemical cellPhotoelectrochemical cell
Photoelectrochemical cells or PECs are solar cells which generate electrical energy from light, including visible light. Some photoelectrochemical cells simply produce electrical energy, while others produce hydrogen in a process similar to the electrolysis of water.-Photogeneration cell:In this...
(PEC) process which is also named artificial photosynthesis
Artificial photosynthesis
Artificial photosynthesis is a chemical process that replicates the natural process of photosynthesis, a process that converts sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term is commonly used to refer to any scheme for capturing and storing the energy from sunlight in...
. Research aimed toward developing higher-efficiency multi-junction cell technology is underway by the photovoltaic industry. If this process is assisted by photocatalysts suspended directly in water instead of using photovoltaic and an electrolytic system, the reaction is in just one step, which can improve efficiency.
Concentrating solar thermal
Very high temperatures are required to dissociate water into hydrogen and oxygen. A catalyst is required to make the process operate at feasible temperatures. Heating the water can be achieved through the use of concentrating solar power. Hydrosol-2Hydrosol-2
HYDROSOL is series of European Union funded projects for the promotion of renewable energy...
is a 100-kilowatt pilot plant at the Plataforma Solar de Almería
Plataforma Solar de Almería
The Plataforma Solar de Almería is a center for the exploration of the solar energy, situated in the Province of Almería.-History:It was founded in the early 1980s and run by the centro de Investigaciones Energéticas, Medioambientales y Tecnológicas , its location is on the edge of the Tabernas...
in Spain
Spain
Spain , officially the Kingdom of Spain languages]] under the European Charter for Regional or Minority Languages. In each of these, Spain's official name is as follows:;;;;;;), is a country and member state of the European Union located in southwestern Europe on the Iberian Peninsula...
which uses sunlight to obtain the required 800 to 1,200 °C to heat water. Hydrosol II has been in operation since 2008. The design of this 100-kilowatt pilot plant is based on a modular concept. As a result, it may be possible that this technology could be readily scaled up to the megawatt range by multiplying the available reactor units and by connecting the plant to heliostat
Heliostat
A heliostat is a device that includes a mirror, usually a plane mirror, which turns so as to keep reflecting sunlight toward a predetermined target, compensating for the sun's apparent motions in the sky. The target may be a physical object, distant from the heliostat, or a direction in space...
fields (fields of sun-tracking mirrors) of a suitable size.
Photoelectrocatalytic production
A method studied by Thomas Nann and his team at the University of East Anglia consists of a gold electrode covered in layers of indium phosphide (InP) nanoparticles. They introduced an iron-sulfur complex into the layered arrangement, which when submerged in water and irradiated with light under a small electric current, produced hydrogen with an efficiency of 60%.Thermochemical production
There are more than 352 thermochemical cycles which can be used for water splittingWater splitting
Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in...
, around a dozen of these cycles such as the iron oxide cycle
Iron oxide cycle
The iron oxide cycle is a two-step thermochemical cycle proposed for use for hydrogen production.-Process description:The thermochemical two-step water splitting process uses redox systems...
, cerium(IV) oxide-cerium(III) oxide cycle
Cerium(IV) oxide-cerium(III) oxide cycle
The cerium oxide–cerium oxide cycle or CeO2/Ce2O3 cycle is a two step thermochemical process based on cerium oxide and cerium oxide for hydrogen production...
, zinc zinc-oxide cycle, sulfur-iodine cycle
Sulfur-iodine cycle
The sulfur–iodine cycle is a three-step thermochemical cycle used to produce hydrogen.The S–I cycle consists of three chemical reactions whose net reactant is water and whose net products are hydrogen and oxygen. All other chemicals are recycled...
, copper-chlorine cycle
Copper-chlorine cycle
The copper–chlorine cycle is a four-step thermochemical cycle. It has a maximum temperature requirement of about 530 degrees Celsius. The Cu–Cl cycle is one of the prominent thermochemical cycles under development within the Generation IV International Forum...
and hybrid sulfur cycle
Hybrid sulfur cycle
The hybrid sulfur cycle is a two-step water-splitting process intended to be used for hydrogen production. Based on sulfur oxidation and reduction, it is classified as a hybrid thermochemical cycle because it uses an electrochemical reaction for one of the two steps...
are under research and in testing phase to produce hydrogen and oxygen from water and heat without using electricity. These processes can be more efficient than high-temperature electrolysis, typical in the range from 35 % - 49 % LHV efficiency. Thermochemical production of hydrogen using chemical energy from coal or natural gas is generally not considered, because the direct chemical path is more efficient.
None of the thermochemical hydrogen production processes have been demonstrated at production levels, although several have been demonstrated in laboratories.
Storage
Although molecular hydrogen has very high energy density on a mass basis, partly because of its low molecular weight, as a gas at ambient conditions it has very low energy density by volume. If it is to be used as fuel stored on board the vehicle, pure hydrogen gas must be pressurized or liquefied to provide sufficient driving range. Increasing gas pressure improves the energy density by volume, making for smaller, but not lighter container tanks (see pressure vesselPressure vessel
A pressure vessel is a closed container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.The pressure differential is dangerous and many fatal accidents have occurred in the history of their development and operation. Consequently, their design,...
). Achieving higher pressures necessitates greater use of external energy to power the compression. Alternatively, higher volumetric energy density liquid hydrogen
Liquid hydrogen
Liquid hydrogen is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.To exist as a liquid, H2 must be pressurized above and cooled below hydrogen's Critical point. However, for hydrogen to be in a full liquid state without boiling off, it needs to be...
or slush hydrogen
Slush hydrogen
Slush hydrogen is a combination of liquid hydrogen and solid hydrogen at the triple point with a lower temperature and a higher density than liquid hydrogen. It is formed by bringing liquid hydrogen down to nearly the melting point that increases density by 16–20% as compared to liquid hydrogen...
may be used. However, liquid hydrogen is cryogenic and boils at 20.268 K (–252.882 °C or –423.188 °F). Cryogenic storage cuts weight but requires large liquification
Liquification
In physics, to liquefy means to turn something into the liquid state.-In Geology:In geology, liquefaction refers to the process by which saturated, unconsolidated sediments are transformed into a substance that acts like a liquid.Earthquakes can cause soil liquefaction where loosely packed,...
energies. The liquefaction process, involving pressurizing and cooling steps, is energy intensive. The liquefied hydrogen has lower energy density by volume than gasoline by approximately a factor of four, because of the low density of liquid hydrogen — there is actually more hydrogen in a liter of gasoline (116 grams) than there is in a liter of pure liquid hydrogen (71 grams). Liquid hydrogen storage tanks
Hydrogen tank
A Hydrogen tank is used for hydrogen storage. The first type IV hydrogen tanks for compressed hydrogen at 700 Bar were demonstrated in 2001, the first fuel cell vehicles on the road with type IV tanks are the Toyota FCHV, Mercedes-Benz F-Cell and the HydroGen4.At the hydrogen station Hamburg...
must also be well insulated to minimize boil off. Ice may form around the tank and help corrode it further if the liquid hydrogen tank insulation fails.
The mass of the tanks needed for compressed hydrogen
Compressed hydrogen
Compressed hydrogen is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar and 700 bar is used for mobile hydrogen storage in hydrogen vehicles...
reduces the fuel economy of the vehicle. Because it is a small molecule, hydrogen tends to diffuse through any liner material intended to contain it, leading to the embrittlement
Hydrogen embrittlement
Hydrogen embrittlement is the process by which various metals, most importantly high-strength steel, become brittle and fracture following exposure to hydrogen...
, or weakening, of its container.
Distinct from storing molecular hydrogen, hydrogen can be stored as a chemical hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
or in some other hydrogen-containing compound. Hydrogen gas is reacted with some other materials to produce the hydrogen storage material, which can be transported relatively easily. At the point of use the hydrogen storage material can be made to decompose, yielding hydrogen gas. As well as the mass and volume density problems associated with molecular hydrogen storage, current barriers to practical storage schemes stem from the high pressure and temperature conditions needed for hydride formation and hydrogen release. For many potential systems hydriding and dehydriding kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
and heat management are also issues that need to be overcome. A French company McPhy Energy http://www.mcphy.com is developing the first industrial product, based on Magnesium Hydrate, already sold to some major clients such as Iwatani and ENEL.
A third approach is to absorb molecular hydrogen into a solid storage material. Unlike in the hydrides mentioned above, the hydrogen does not dissociate/recombine upon charging/discharging the storage system, and hence does not suffer from the kinetic limitations of many hydride storage systems. Hydrogen densities similar to liquefied hydrogen can be achieved with appropriate absorption media. Some suggested absorbers include MOFs
Metal-organic framework
Metal-Organic Frameworks are crystalline compounds consisting of metal ions or clusters coordinated to often rigid organic molecules to form one-, two-, or three-dimensional structures that can be porous. In some cases, the pores are stable to elimination of the guest molecules and can be used for...
, nanostructure
Nanostructure
A nanostructure is an object of intermediate size between molecular and microscopic structures.In describing nanostructures it is necessary to differentiate between the number of dimensions on the nanoscale. Nanotextured surfaces have one dimension on the nanoscale, i.e., only the thickness of the...
d carbons (including CNTs
Carbon nanotube
Carbon nanotubes are allotropes of carbon with a cylindrical nanostructure. Nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1, significantly larger than for any other material...
) and hydrogen clathrate hydrate.
The most common method of on board hydrogen storage in today's demonstration vehicles is as a compressed gas at pressures of roughly 700 bar (70 MPa
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
).
Underground hydrogen storage
Underground hydrogen storage
Underground hydrogen storage is the practice of hydrogen storage in underground caverns, salt domes and depleted oil/gas fields. Large quantities of gaseous hydrogen have been stored in underground caverns by ICI for many years without any difficulties...
is the practice of hydrogen storage
Hydrogen storage
Hydrogen storage describes the methods for storing H2 for subsequent use. The methods span many approaches, including high pressures, cryogenics, and chemical compounds that reversibly release H2 upon heating...
in underground cave
Cave
A cave or cavern is a natural underground space large enough for a human to enter. The term applies to natural cavities some part of which is in total darkness. The word cave also includes smaller spaces like rock shelters, sea caves, and grottos.Speleology is the science of exploration and study...
rns, salt dome
Salt dome
A salt dome is a type of structural dome formed when a thick bed of evaporite minerals found at depth intrudes vertically into surrounding rock strata, forming a diapir....
s and depleted oil and gas fields. Large quantities of gaseous hydrogen are stored in underground caverns by ICI
Imperial Chemical Industries
Imperial Chemical Industries was a British chemical company, taken over by AkzoNobel, a Dutch conglomerate, one of the largest chemical producers in the world. In its heyday, ICI was the largest manufacturing company in the British Empire, and commonly regarded as a "bellwether of the British...
for many years without any difficulties. The storage of large quantities of hydrogen underground can function as grid energy storage
Grid energy storage
Grid energy storage refers to the methods used to store electricity on a large scale within an electrical power grid. Electrical energy is stored during times when production exceeds consumption and the stores are used at times when consumption exceeds production...
which is essential for the hydrogen economy.
Alternative storage proposal
It has been proposed in a hypothetical renewable energy dominated energy system to use the excess electricity generated by wind, solar photovoltaic, hydro, marine currents and others to make methane (natural gas) by electrolysis of water. The methane could then be injected into the existing gas network to generate electricity and heat on demand to overcome low points of renewable energy production. The process described would be to create hydrogen (which could partly be used directly in fuel cells) and the addition of carbon dioxide CO2 (Sabatier process) to create methane as follows:
CO2 + 4H2 → CH4 + 2H2O
Infrastructure

Hydrogen pipeline transport
Hydrogen pipeline transport is a transportation of hydrogen through a pipe as part of the hydrogen infrastructure.-Economics:Hydrogen pipeline transport is used to transport hydrogen from the point of production or delivery to the point of demand...
and hydrogen-equipped filling stations like those found on a hydrogen highway
Hydrogen highway
A hydrogen highway is a chain of hydrogen-equipped filling stations and other infrastructure along a road or highway which allow hydrogen powered cars to travel. It is an element of the hydrogen infrastructure that is generally assumed to be a pre-requisite for mass utilization of hydrogen cars....
. Hydrogen stations which were not situated near a hydrogen pipeline would get supply via hydrogen tank
Hydrogen tank
A Hydrogen tank is used for hydrogen storage. The first type IV hydrogen tanks for compressed hydrogen at 700 Bar were demonstrated in 2001, the first fuel cell vehicles on the road with type IV tanks are the Toyota FCHV, Mercedes-Benz F-Cell and the HydroGen4.At the hydrogen station Hamburg...
s, compressed hydrogen tube trailers, liquid hydrogen trailer
Liquid hydrogen trailer
A liquid hydrogen trailer is a trailer designed to carry cryogenic liquid hydrogen on roads being pulled by a powered vehicle. The largest such vehicles are similar to railroad tanktainers which are also designed to carry liquefied loads. Liquid hydrogen trailers tend to be large; they are...
s, liquid hydrogen tank trucks or dedicated onsite production.
Because of hydrogen embrittlement
Hydrogen embrittlement
Hydrogen embrittlement is the process by which various metals, most importantly high-strength steel, become brittle and fracture following exposure to hydrogen...
of steel, and corrosion natural gas pipes require internal coatings or replacement in order to convey hydrogen. Techniques are well-known; over 700 miles of hydrogen pipeline
Hydrogen piping
Hydrogen piping, in industrial settings, is a system of pipes used to move hydrogen. Due to issues with hydrogen embrittlement, and corrosion, materials for hydrogen pipes must be carefully selected...
currently exist in the United States. Although expensive, pipelines are the cheapest way to move hydrogen. Hydrogen gas piping is routine in large oil-refineries, because hydrogen is used to hydrocrack fuels from crude oil.
Hydrogen piping can in theory be avoided in distributed systems of hydrogen production, where hydrogen is routinely made on site using medium or small-sized generators which would produce enough hydrogen for personal use or perhaps a neighborhood. In the end, a combination of options for hydrogen gas distribution may succeed.
While millions of tons of elemental hydrogen are distributed around the world each year in various ways, bringing hydrogen to individual consumers would require an evolution of the fuel infrastructure. For example, according to GM, 70% of the U.S. population lives near a hydrogen-generating facility but has little public access to that hydrogen. The same study however, shows that building the infrastructure in a systematic way is much more doable and affordable than most people think. For example, one article has noted that hydrogen stations could be put within every 10 miles in metro Los Angeles, and on the highways between LA and neighboring cities like Palm Springs, Las Vegas, San Diego and Santa Barbara, for the cost of a Starbuck's latte for every one of the 15 million residents living in these areas.
A key tradeoff: centralized vs. distributed production
In a future full hydrogen economy, primary energy sources and feedstock would be used to produce hydrogen gas as stored energy for use in various sectors of the economy. Producing hydrogen from primary energy sources other than coal, oil, and natural gas, would result in lower production of the greenhouse gases characteristic of the combustion of these fossil energy resources.One key feature of a hydrogen economy would be that in mobile applications (primarily vehicular transport) energy generation and use could be decoupled. The primary energy source would need no longer travel with the vehicle, as it currently does with hydrocarbon fuels. Instead of tailpipes creating dispersed emissions, the energy (and pollution) could be generated from point sources such as large-scale, centralized facilities with improved efficiency. This would allow the possibility of technologies such as carbon sequestration, which are otherwise impossible for mobile applications. Alternatively, distributed energy generation
Distributed generation
Distributed generation, also called on-site generation, dispersed generation, embedded generation, decentralized generation, decentralized energy or distributed energy, generates electricity from many small energy sources....
schemes (such as small scale renewable energy sources) could be used, possibly associated with hydrogen stations.
Aside from the energy generation, hydrogen production could be centralized, distributed or a mixture of both. While generating hydrogen at centralized primary energy plants promises higher hydrogen production efficiency, difficulties in high-volume, long range hydrogen transportation (due to factors such as hydrogen damage
Hydrogen damage
Hydrogen damage is the generic name given to a large number of metal degradation processes due to interaction with hydrogen.Hydrogen is present practically everywhere, in the atmosphere, several kilometres above the earth and inside the earth. Engineering materials are exposed to hydrogen and they...
and the ease of hydrogen diffusion through solid materials) makes electrical energy distribution attractive within a hydrogen economy. In such a scenario, small regional plants or even local filling stations could generate hydrogen using energy provided through the electrical distribution grid. While hydrogen generation efficiency is likely to be lower than for centralized hydrogen generation, losses in hydrogen transport could make such a scheme more efficient in terms of the primary energy used per kilogram of hydrogen delivered to the end user.
The proper balance between hydrogen distribution and long-distance electrical distribution is one of the primary questions that arises about the hydrogen economy.
Again the dilemmas of production sources and transportation of hydrogen can now be overcome using on site (home, business, or fuel station) generation of hydrogen from off grid renewable sources.http://itm-power.com.
Distributed electrolysis
Distributed electrolysis would bypass the problems of distributing hydrogen by distributing electricity instead. It would use existing electrical networks to transport electricity to small, on-site electrolysers located at filling stations. However, accounting for the energy used to produce the electricity and transmission losses would reduce the overall efficiency.Natural gas combined cycle power plants, which account for almost all construction of new electricity generation plants in the United States, generate electricity at efficiencies of 60 percent or greater. Increased demand for electricity, whether due to hydrogen cars or other demand, would have the marginal impact of adding new combined cycle power plants. On this basis, distributed production of hydrogen would be roughly 40% efficient. However, if the marginal impact is referred to today's power grid, with an efficiency of roughly 40% owing to its mix of fuels and conversion methods, the efficiency of distributed hydrogen production would be roughly 25%.
The distributed production of hydrogen in this fashion would be expected to generate air emissions of pollutants and carbon dioxide at various points in the supply chain, e.g., electrolysis, transportation and storage. Such externalities as pollution must be weighed against the potential advantages of a hydrogen economy.
Fuel cells as alternative to internal combustion
One of the main offerings of a hydrogen economy is that the fuel can replace the fossil fuel burned in internal combustion engineInternal combustion engine
The internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine...
s and turbine
Turbine
A turbine is a rotary engine that extracts energy from a fluid flow and converts it into useful work.The simplest turbines have one moving part, a rotor assembly, which is a shaft or drum with blades attached. Moving fluid acts on the blades, or the blades react to the flow, so that they move and...
s as the primary way to convert chemical energy into kinetic or electrical energy; hereby eliminating greenhouse gas emissions and pollution from that engine.
Although hydrogen can be used in conventional internal combustion engines, fuel cells,
being electrochemical, have a theoretical efficiency advantage over heat engines. Fuel cells are more expensive to produce than common internal combustion engines, but are becoming cheaper as new technologies and production systems develop.
Some types of fuel cells work with hydrocarbon fuels, while all can be operated on pure hydrogen. In the event that fuel cells become price-competitive with internal combustion engines and turbines, large gas-fired power plants could adopt this technology.
Hydrogen gas must be distinguished as "technical-grade" (five nines pure), which is suitable for applications such as fuel cells, and "commercial-grade", which has carbon- and sulfur-containing impurities, but which can be produced by the much cheaper steam-reformation process. Fuel cells require high purity hydrogen because the impurities would quickly degrade the life of the fuel cell stack.
Much of the interest in the hydrogen economy concept is focused on the use of fuel cells to power electric car
Čar
Čar is a village in the municipality of Bujanovac, Serbia. According to the 2002 census, the town has a population of 296 people.-References:...
s. Current Hydrogen fuel cells suffer from a low power-to-weight ratio
Power-to-weight ratio
Power-to-weight ratio is a calculation commonly applied to engines and mobile power sources to enable the comparison of one unit or design to another. Power-to-weight ratio is a measurement of actual performance of any engine or power sources...
. Fuel cells are much more efficient than internal combustion engines, and produce no harmful emissions. If a practical method of hydrogen storage
Hydrogen storage
Hydrogen storage describes the methods for storing H2 for subsequent use. The methods span many approaches, including high pressures, cryogenics, and chemical compounds that reversibly release H2 upon heating...
is introduced, and fuel cells become cheaper, they can be economically viable to power hybrid
Hybrid vehicle
A hybrid vehicle is a vehicle that uses two or more distinct power sources to move the vehicle. The term most commonly refers to hybrid electric vehicles , which combine an internal combustion engine and one or more electric motors.-Power:...
fuel cell/battery vehicles, or purely fuel cell-driven ones. The economic viability of fuel cell powered vehicles will improve as the hydrocarbon fuels used in internal combustion engines become more expensive, because of the depletion of easily accessible reserves or economic accounting of environmental impact through such measures as carbon tax
Carbon tax
A carbon tax is an environmental tax levied on the carbon content of fuels. It is a form of carbon pricing. Carbon is present in every hydrocarbon fuel and is released as carbon dioxide when they are burnt. In contrast, non-combustion energy sources—wind, sunlight, hydropower, and nuclear—do not...
es.
Other fuel cell technologies based on the exchange of metal ions (i.e. zinc-air fuel cells
Zinc-air battery
Zinc–air batteries , and zinc–air fuel cells, are electro-chemical batteries powered by oxidizing zinc with oxygen from the air. These batteries have high energy densities and are relatively inexpensive to produce...
) are typically more efficient at energy conversion than hydrogen fuel cells, but the widespread use of any electrical energy → chemical energy → electrical energy systems would necessitate the production of electricity.
Efficiency as an automotive fuel
Hydrogen has been called one of the least efficient and most expensive possible replacements for gasoline (petrol) in terms of reducing greenhouse gases; other technologies may be less expensive and more quickly implemented. A comprehensive study of hydrogen in transportation applications has found that "there are major hurdles on the path to achieving the vision of the hydrogen economy; the path will not be simple or straightforward". The Ford Motor CompanyFord Motor Company
Ford Motor Company is an American multinational automaker based in Dearborn, Michigan, a suburb of Detroit. The automaker was founded by Henry Ford and incorporated on June 16, 1903. In addition to the Ford and Lincoln brands, Ford also owns a small stake in Mazda in Japan and Aston Martin in the UK...
has dropped its plans to develop hydrogen cars, stating that "The next major step in Ford’s plan is to increase over time the volume of electrified vehicles".
An accounting of the energy utilized during a thermodynamic process, known as an energy balance, can be applied to automotive fuels. With today's technology, the manufacture of hydrogen via steam reforming
Steam reforming
Fossil fuel reforming is a method of producing hydrogen or other useful products from fossil fuels such as natural gas. This is achieved in a processing device called a reformer which reacts steam at high temperature with the fossil fuel. The steam methane reformer is widely used in industry to...
can be accomplished with a thermal efficiency of 75 to 80 percent. Additional energy will be required to liquefy or compress the hydrogen, and to transport it to the filling station via truck or pipeline. The energy that must be utilized per kilogram to produce, transport and deliver hydrogen (i.e., its well-to-tank energy use) is approximately 50 megajoules using technology available in 2004. Subtracting this energy from the enthalpy of one kilogram of hydrogen, which is 141 megajoules, and dividing by the enthalpy, yields a thermal energy efficiency of roughly 60%. Gasoline, by comparison, requires less energy input, per gallon, at the refinery, and comparatively little energy is required to transport it and store it owing to its high energy density per gallon at ambient temperatures. Well-to-tank, the supply chain for gasoline is roughly 80% efficient (Wang, 2002). The most efficient distribution however is electrical
Electrical power industry
The electric power industry provides the production and delivery of electric energy, often known as power, or electricity, in sufficient quantities to areas that need electricity through a grid connection. The grid distributes electrical energy to customers...
, which is typically 95% efficient. Electric vehicles
Electric car
An electric car is an automobile which is propelled by electric motor, using electrical energy stored in batteries or another energy storage device. Electric cars were popular in the late-19th century and early 20th century, until advances in internal combustion engine technology and mass...
are typically 3 to 4 times as efficient as hydrogen powered vehicles
Hydrogen vehicle
A hydrogen vehicle is a vehicle that uses hydrogen as its onboard fuel for motive power. Hydrogen vehicles include hydrogen fueled space rockets, as well as automobiles and other transportation vehicles...
.

Hydrogen vehicle
A hydrogen vehicle is a vehicle that uses hydrogen as its onboard fuel for motive power. Hydrogen vehicles include hydrogen fueled space rockets, as well as automobiles and other transportation vehicles...
s compared to other vehicles in the Norwegian energy system indicates that hydrogen fuel-cell vehicles tend to be about a third as efficient as EVs when electrolysis is used, with hydrogen Internal Combustion Engines (ICE) being barely a sixth as efficient. Even in the case where hydrogen fuel cells get their hydrogen from natural gas reformation rather than electrolysis, and EVs get their power from a natural gas power plant, the EVs still come out ahead 35% to 25% (and only 13% for a H2 ICE). This compares to 14% for a gasoline ICE, 27% for a gasoline ICE hybrid, and 17% for a diesel ICE, also on a well-to-wheels basis.
Hydrogen safety
Hydrogen has one of the widest explosive/ignition mix range with air of all the gases with few exceptions such as acetyleneAcetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
, silane
Silane
Silane is a toxic, extremely flammable chemical compound with chemical formula SiH4. In 1857, the German chemists and Friedrich Woehler discovered silane among the products formed by the action of hydrochloric acid on aluminum silicide, which they had previously prepared...
, and ethylene oxide
Ethylene oxide
Ethylene oxide, also called oxirane, is the organic compound with the formula . It is a cyclic ether. This means that it is composed of two alkyl groups attached to an oxygen atom in a cyclic shape . This colorless flammable gas with a faintly sweet odor is the simplest epoxide, a three-membered...
. That means that whatever the mix proportion between air and hydrogen, a hydrogen leak will most likely lead to an explosion, not a mere flame, when a flame or spark ignites the mixture. This makes the use of hydrogen particularly dangerous in enclosed areas such as tunnels or underground parking. Pure hydrogen-oxygen flames burn in the ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
color range and are nearly invisible to the naked eye, so a flame detector
Flame detector
There are several types of flame detector. The optical flame detector is a detector that uses optical sensors to detect flames. There are also ionisation flame detectors, which use current flow in the flame to detect flame presence, and thermocouple flame detectors.-Ultraviolet:Ultraviolet ...
is needed to detect if a hydrogen leak is burning. Hydrogen is odorless and leaks cannot be detected by smell.
Hydrogen codes and standards are code
Code (law)
A code is a type of legislation that purports to exhaustively cover a complete system of laws or a particular area of law as it existed at the time the code was enacted, by a process of codification. Though the process and motivations for codification are similar in common law and civil law...
s and standards for hydrogen fuel cell vehicle
Fuel cell vehicle
A Fuel cell vehicle or Fuel Cell Electric Vehicle is a type of hydrogen vehicle which uses a fuel cell to produce electricity, powering its on-board electric motor...
s, stationary fuel cell applications and portable fuel cell applications
Portable fuel cell applications
Fuel cell applications are stationary fuel cell applications and portable fuel cell plications...
. There are codes and standards for the safe handling and storage of hydrogen, for example the Standard for the installation of stationary fuel cell power systems from the National Fire Protection Association
National Fire Protection Association
The National Fire Protection Association is a United States trade association that creates and maintains private, copywrited, standards and codes for usage and adoption by local governments...
.
Codes and standards have repeatedly been identified as a major institutional barrier to deploying hydrogen technologies
Hydrogen technologies
Hydrogen technologies are technologies that relate to the production and use of hydrogen. Hydrogen technologies are applicable for many uses....
and developing a hydrogen economy. To enable the commercialization of hydrogen in consumer products, new model building codes and equipment and other technical standards are developed and recognized by federal, state, and local governments.
One of the measures on the roadmap is to implement higher safety standards like early leak detection with hydrogen sensors. The Canadian Hydrogen Safety Program concluded that hydrogen fueling is as safe as, or safer than, CNG fueling. The European Commission has funded the first higher educational program in the world in hydrogen safety engineering at the University of Ulster
University of Ulster
The University of Ulster is a multi-campus, co-educational university located in Northern Ireland. It is the largest single university in Ireland, discounting the federal National University of Ireland...
. It is expected that the general public will be able to use hydrogen technologies in everyday life with at least the same level of safety and comfort as with today's fossil fuels.
Environmental concerns
There are many concerns regarding the environmental effects of the manufacture of hydrogen. Hydrogen is made either by electrolysis of waterElectrolysis of water
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to an electric current being passed through the water.-Principle:...
, or by fossil fuel reforming. Reforming a fossil fuel leads to a higher emissions of carbon dioxide compared with direct use of the fossil fuel in an internal combustion engine. Similarly, if hydrogen is produced by electrolysis from fossil-fuel powered generators, increased carbon dioxide is emitted in comparison with direct use of the fossil fuel.
Using renewable energy source to generate hydrogen by electrolysis would require greater energy input than direct use of the renewable energy to operate electric vehicles, because of the extra conversion stages and losses in distribution.
Like any internal combustion engine, an ICE running on hydrogen may produce nitrous oxides and other pollutants. Air input into the combustion cylinder is approximately 78% nitrogen, and the N2 molecule has a binding energy
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
of approximately 226 kilocalories per mole. The hydrogen reaction has sufficient energy to break this bond and produce unwanted components such as nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
(HNO3), and hydrogen cyanide gas (HCN), both toxic byproducts. Nitrogen compound emissions from internal combustion engines are a root cause of smog.
Hydrogen as transportation fuel, however, is mainly used for fuel cells that do not produce greenhouse gas emission, but water.
There have also been some concerns over possible problems related to hydrogen gas leakage. Molecular hydrogen leaks slowly from most containment vessels. It has been hypothesized that if significant amounts of hydrogen gas (H2) escape, hydrogen gas may, because of ultraviolet radiation, form free radicals (H) in the stratosphere. These free radicals would then be able to act as catalysts for ozone depletion
Ozone depletion
Ozone depletion describes two distinct but related phenomena observed since the late 1970s: a steady decline of about 4% per decade in the total volume of ozone in Earth's stratosphere , and a much larger springtime decrease in stratospheric ozone over Earth's polar regions. The latter phenomenon...
. A large enough increase in stratospheric hydrogen from leaked H2 could exacerbate the depletion process. However, the effect of these leakage problems may not be significant. The amount of hydrogen that leaks today is much lower (by a factor of 10–100) than the estimated 10–20% figure conjectured by some researchers; for example, in Germany
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
, the leakage rate is only 0.1% (less than the natural gas leak rate of 0.7%). At most, such leakage would likely be no more than 1–2% even with widespread hydrogen use, using present technology.
Costs
When evaluating costs, fossil fuels are generally used as the reference. The energy content of these fuels is not a product of human effort and so has no cost assigned to it. Only the extraction, refining, transportation and production costs are considered. On the other hand, the energy content of a unit of hydrogen fuel must be manufactured, and so has a significant cost, on top of all the costs of refining, transportation, and distribution. Systems which use renewably generated electricity more directly, for example in trolleybusTrolleybus
A trolleybus is an electric bus that draws its electricity from overhead wires using spring-loaded trolley poles. Two wires and poles are required to complete the electrical circuit...
es, or in battery electric vehicle
Battery electric vehicle
A battery electric vehicle, or BEV, is a type of electric vehicle that uses chemical energy stored in rechargeable battery packs. BEVs use electric motors and motor controllers instead of, or in addition to, internal combustion engines for propulsion.A battery-only electric vehicle or...
s may have a significant economic advantage because there are fewer conversion processes required between primary energy source and point of use.
The barrier to lowering the price of high purity hydrogen is a cost of more than 35 kWh of electricity used to generate each kilogram of hydrogen gas.
Demonstrated advances in electrolyzer and fuel cell technology by ITM Power are claimed to have made significant in-roads into addressing the cost of electrolysing water to make hydrogen. Cost reduction would make hydrogen from off-grid renewable sources economic for refueling vehicles.
Hydrogen pipelines are more expensive than even long-distance electric lines. Hydrogen is about three times bulkier in volume than natural gas for the same enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
. Hydrogen accelerates the cracking of steel (hydrogen embrittlement
Hydrogen embrittlement
Hydrogen embrittlement is the process by which various metals, most importantly high-strength steel, become brittle and fracture following exposure to hydrogen...
), which increases maintenance costs, leakage rates, and material costs. The difference in cost is likely to expand with newer technology: wires suspended in air can use higher voltage with only marginally increased material costs, but higher pressure pipes require proportionally more material.
Setting up a hydrogen economy would require huge investments in the infrastructure to store and distribute hydrogen to vehicles. In contrast, battery electric vehicle
Battery electric vehicle
A battery electric vehicle, or BEV, is a type of electric vehicle that uses chemical energy stored in rechargeable battery packs. BEVs use electric motors and motor controllers instead of, or in addition to, internal combustion engines for propulsion.A battery-only electric vehicle or...
s, which are already publicly available, would not necessitate immediate expansion of the existing infrastructure for electricity transmission and distribution. Power plant capacity that now goes unused at night could be used for recharging electric vehicles. A study conducted by the Pacific Northwest National Laboratory for the US Department of Energy in December 2006 found that the idle off-peak grid capacity in the US would be sufficient to power 84% of all vehicles in the US if they all were immediately replaced with electric vehicles.
Different production methods each have differing associated investment and marginal costs. The energy and feedstock could originate from a multitude of sources i.e. natural gas, nuclear, solar, wind, biomass, coal, other fossil fuels, and geothermal.
Natural Gas at Small Scale: Uses steam reformation. Requires 15.9 Mcuft of gas, which, if produced by small 500 kg/day reformers at the point of dispensing (i.e., the filling station), would equate to 777,000 reformers costing $1 trillion dollars and producing 150 million tons of hydrogen gas annually. Obviates the need for distribution infrastructure dedicated to hydrogen. $3.00 per GGE (Gallons of Gasoline Equivalent)
Nuclear: Provides energy for electrolysis of water. Would require 240,000 tons of unenriched uranium — that's 2,000 600-megawatt power plants, which would cost $840 billion, or about $2.50 per GGE.
Solar: Provides energy for electrolysis of water. Would require 2,500 kWh of sun per square meter, 113 million 40-kilowatt systems, which would cost $22 trillion, or about $9.50 per GGE.
Wind: Provides energy for electrolysis of water. At 7 meters per second average wind speed, it would require 1 million 2-MW wind turbines, which would cost $3 trillion dollars, or about $3.00 per GGE.
Biomass: Gasification plants would produce gas with steam reformation. 1.5 billion tons of dry biomass, 3,300 plants which would require 113.4 million acres (460,000 km²) of farm to produce the biomass. $565 billion dollars in cost, or about $1.90 per GGE
Coal: FutureGen plants use coal gasification then steam reformation. Requires 1 billion tons of coal or about 1,000 275-megawatt plants with a cost of about $500 billion, or about $1 per GGE.
- DOE Cost targets
Examples and pilot programs
Several domestic U.S.United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
automobile
Automobile
An automobile, autocar, motor car or car is a wheeled motor vehicle used for transporting passengers, which also carries its own engine or motor...
manufactures have committed to develop vehicles using hydrogen. The distribution of hydrogen for the purpose of transportation is currently being tested around the world, particularly in Portugal
Portugal
Portugal , officially the Portuguese Republic is a country situated in southwestern Europe on the Iberian Peninsula. Portugal is the westernmost country of Europe, and is bordered by the Atlantic Ocean to the West and South and by Spain to the North and East. The Atlantic archipelagos of the...
, Iceland
Iceland
Iceland , described as the Republic of Iceland, is a Nordic and European island country in the North Atlantic Ocean, on the Mid-Atlantic Ridge. Iceland also refers to the main island of the country, which contains almost all the population and almost all the land area. The country has a population...
, Norway
Hynor
The HyNor hydrogen highway in Norway was established in 2003 and is part of the Scandinavian hydrogen highway partnership. The highway is part of the Norwegian hydrogen infrastructure, and several hydrogen refuelling stations have been built along the route....
, Denmark
Hydrogen link network
The Hydrogen link network in Denmark was established in 2005 by the Nordic Transportpolitical Network to form a hydrogen highway with hydrogen Sweden and hynor as part of the Scandinavian hydrogen highway partnership....
, Germany
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
, California
California Hydrogen Highway
The California Hydrogen Highway is a series of hydrogen refueling stations in California. These stations are used to refuel hydrogen vehicles such as fuel cell vehicles and hydrogen combustion vehicles....
, Japan and Canada
BC hydrogen highway
The BC Hydrogen Highway is a hydrogen highway planned to link Vancouver and Whistler, host city and alpine venue of the 2010 Winter Olympics. It is targeted for full implementation by the start of the games. Currently seven hydrogen fueling stations are being planned, located in Victoria, Richmond,...
, but the cost is very high.
Some hospitals have installed combined electrolyzer-storage-fuel cell units for local emergency power. These are advantageous for emergency use because of their low maintenance requirement and ease of location compared to internal combustion driven generators.
Iceland
Iceland
Iceland , described as the Republic of Iceland, is a Nordic and European island country in the North Atlantic Ocean, on the Mid-Atlantic Ridge. Iceland also refers to the main island of the country, which contains almost all the population and almost all the land area. The country has a population...
has committed to becoming the world's first hydrogen economy by the year 2050. Iceland is in a unique position. Presently, it imports all the petroleum products necessary to power its automobiles and fishing fleet
Fishing fleet
A fishing fleet is an aggregate of commercial fishing vessels. The term may be used of all vessels operating out of a particular port, all vessels engaged in a particular type of fishing , or all fishing vessels of a country or region.Although fishing vessels are not formally organized as if they...
. Iceland has large geothermal resources, so much that the local price of electricity actually is lower than the price of the hydrocarbons that could be used to produce that electricity.
Iceland already converts its surplus electricity into exportable goods and hydrocarbon replacements. In 2002, it produced 2,000 tons of hydrogen gas by electrolysis—primarily for the production of ammonia (NH3) for fertilizer. Ammonia is produced, transported, and used throughout the world, and 90% of the cost of ammonia is the cost of the energy to produce it. Iceland is also developing an aluminium -smelting industry. Aluminium costs are primarily driven by the cost of the electricity to run the smelters. Either of these industries could effectively export all of Iceland's potential geothermal electricity
Geothermal electricity
Geothermal electricity is electricity generated from geothermal energy.Technologies in use include dry steam power plants, flash steam power plants and binary cycle power plants...
.
Neither industry directly replaces hydrocarbons. Reykjavík
Reykjavík
Reykjavík is the capital and largest city in Iceland.Its latitude at 64°08' N makes it the world's northernmost capital of a sovereign state. It is located in southwestern Iceland, on the southern shore of Faxaflói Bay...
, Iceland, had a small pilot fleet of city buses running on compressed hydrogen, and research on powering the nation's fishing fleet with hydrogen is under way. For more practical purposes, Iceland might process imported oil with hydrogen to extend it, rather than to replace it altogether.
The Reykjavík buses are part of a larger program, HyFLEET:CUTE, operating hydrogen fueled buses in eight European cities. HyFLEET:CUTE buses also operate in Beijing and Perth (see below).
A pilot project demonstrating a hydrogen economy is operational on the Norwegian
Norway
Norway , officially the Kingdom of Norway, is a Nordic unitary constitutional monarchy whose territory comprises the western portion of the Scandinavian Peninsula, Jan Mayen, and the Arctic archipelago of Svalbard and Bouvet Island. Norway has a total area of and a population of about 4.9 million...
island of Utsira
Utsira
Utsira is a municipality in Rogaland county, Norway. It is part of the traditional district of Haugaland. Utsira was separated from Torvastad on 1 July 1924.The municipality consists of an island located in the North Sea, 18 km west of Haugesund...
. The installation combines wind power
Wind power
Wind power is the conversion of wind energy into a useful form of energy, such as using wind turbines to make electricity, windmills for mechanical power, windpumps for water pumping or drainage, or sails to propel ships....
and hydrogen power. In periods when there is surplus wind energy, the excess power is used for generating hydrogen by electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
. The hydrogen is stored, and is available for power generation in periods when there is little wind.
A joint venture between NREL and Xcel Energy
Xcel Energy
Xcel Energy, Inc. is a public utility company based in Minneapolis, Minnesota, serving customers in Colorado, Michigan, Minnesota, New Mexico, North Dakota, South Dakota, Texas, and Wisconsin. Primary services are electricity and natural gas...
is combining wind power
Wind power
Wind power is the conversion of wind energy into a useful form of energy, such as using wind turbines to make electricity, windmills for mechanical power, windpumps for water pumping or drainage, or sails to propel ships....
and hydrogen power in the same way in Colorado.
In the Netherlands, one fossil fuel power plant (with full carbon capture) is already scheduled to incorporate the generating of hydrogen. The project is called CGEN and the power plant wil be built in the port of Rotterdam.
Hydro
Newfoundland and Labrador Hydro
Newfoundland and Labrador Hydro is a provincial Crown corporation that generates and delivers electricity for Newfoundland and Labrador, Quebec, and the north-eastern areas of the United States. It also delivers voice and data services to customers in some areas...
in Newfoundland and Labrador
Newfoundland and Labrador
Newfoundland and Labrador is the easternmost province of Canada. Situated in the country's Atlantic region, it incorporates the island of Newfoundland and mainland Labrador with a combined area of . As of April 2011, the province's estimated population is 508,400...
are converting the current wind-diesel Power System on the remote island of Ramea into a Wind-Hydrogen Hybrid Power Systems facility.
A similar pilot project on Stuart Island
Stuart Island (Washington)
Stuart Island is one of the San Juan Islands in Washington state, USA, north of San Juan Island and west of Waldron Island. The island is home to two communities of full and part-time residents, a state park, a one-room schoolhouse, and two airstrips .The 2000 census...
uses solar power
Solar power
Solar energy, radiant light and heat from the sun, has been harnessed by humans since ancient times using a range of ever-evolving technologies. Solar radiation, along with secondary solar-powered resources such as wind and wave power, hydroelectricity and biomass, account for most of the available...
, instead of wind power
Wind power
Wind power is the conversion of wind energy into a useful form of energy, such as using wind turbines to make electricity, windmills for mechanical power, windpumps for water pumping or drainage, or sails to propel ships....
, to generate electricity. When excess electricity is available after the batteries are full, hydrogen is generated by electrolysis and stored for later production of electricity by fuel cell.
The UK
United Kingdom
The United Kingdom of Great Britain and Northern IrelandIn the United Kingdom and Dependencies, other languages have been officially recognised as legitimate autochthonous languages under the European Charter for Regional or Minority Languages...
started a fuel cell pilot program in January 2004, the program ran two Fuel cell buses on route 25 in London
London
London is the capital city of :England and the :United Kingdom, the largest metropolitan area in the United Kingdom, and the largest urban zone in the European Union by most measures. Located on the River Thames, London has been a major settlement for two millennia, its history going back to its...
until December 2005, and switched to route RV1 until January 2007.
The Hydrogen Expedition is currently working to create a hydrogen fuel cell-powered ship and using it to circumnavigate the globe, as a way to demonstrate the capability of hydrogen fuel cells.
Western Australia's Department of Planning and Infrastructure currently operates three Daimler Chrysler Citaro fuel cell buses as part of its Sustainable Transport Energy for Perth Fuel Cells Bus Trial in Perth. The buses are operated by Path Transit on regular Transperth public bus routes. The trial began in September 2004 and concluded in September 2006. The buses' fuel cells use a proton exchange membrane system and are supplied with raw hydrogen from a BP refinery in Kwinana, south of Perth. The hydrogen is a byproduct of the refinery's industrial process. The buses are refueled at a station in the northern Perth suburb of Malaga.
The United Nations Industrial Development Organization (UNIDO) and the Turkish Ministry of Energy and Natural Resources
Ministry of Energy and Natural Resources (Turkey)
The Ministry of Energy and Natural Resources is a government ministry office of the Republic of Turkey, responsible for energy and natural resources related affairs in Turkey....
have signed in 2003 a $40M Trust Fund Agreement for the creation in Istanbul of the International Centre for Hydrogen Energy Technologies
International Centre for Hydrogen Energy Technologies
The International Centre for Hydrogen Energy Technologies . Its role is to support, demonstrate and promote viable implementations of hydrogen energy technologies with the aims of enhancing future economic development, particularly in emerging countries...
(UNIDO-ICHET), which started operation in 2004. A hydrogen forklift, a hydrogen cart and a mobile house powered by renewable energies are being demonstrated in UNIDO-ICHET's premises. An uninterruptible power supply system has been working since April 2009 in the headquarters of Istanbul Sea Buses
Ido
Ido is a constructed language created with the goal of becoming a universal second language for speakers of different linguistic backgrounds as a language easier to learn than ethnic languages...
company.
Hydrogen-using alternatives to a fully distributive hydrogen economy
For other energy alternatives, seeHydrogen is simply a method to store and transmit energy. Various alternative energy transmission and storage scenarios which begin with hydrogen production, but do not use it for all parts of the store and transmission infrastructure, may be more economic, in both near and far term. These include:
Ammonia economy
An alternative to gaseous hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
as an energy carrier is to bond it with nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
from the air to produce ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, which can be easily liquefied, transported, and used (directly or indirectly) as a clean and renewable fuel.
Hydrogen production of greenhouse-neutral alcohol
The methanol economy is a synfuel production energy plan which may begin with hydrogen production. Hydrogen in a full "hydrogen economy" was initially suggested as a way to make renewable energyRenewable energy
Renewable energy is energy which comes from natural resources such as sunlight, wind, rain, tides, and geothermal heat, which are renewable . About 16% of global final energy consumption comes from renewables, with 10% coming from traditional biomass, which is mainly used for heating, and 3.4% from...
, in non-polluting form, available to automobiles. However, a theoretical alternative to address the same problem is to produced hydrogen centrally and immediately use it to make liquid fuels from a CO2 source. This would eliminate the requirement to transport and store the hydrogen. The source could be CO2 that is produced by fuel-burning power plants. In order to be greenhouse-neutral, the source for CO2 in such a plan would need to be from air, biomass, or other source of CO2 which is already in, or to be released into, the air.). Direct methanol fuel cells are in commercial use, though they are not presently efficient.
The electrical grid plus synthetic methanol fuel cells
Many of the hybrid strategies described above, using captive hydrogen to generate other more easily usable fuels, might be more effective than hydrogen-production alone. Short term energy storage (meaning the energy is used not long after it has been captured) may be best accomplished with battery or even ultracapacitor storage. Longer term energy storage (meaning the energy is used weeks or months after capture) may be better done with synthetic methane or alcohols, which can be stored indefinitely at relatively low cost, and even used directly in some type of fuel cells, for electric vehicles. These strategies dovetail well with the recent interest in Plug-in Hybrid Electric VehiclesPlug-in hybrid electric vehicle
A plug-in hybrid electric vehicle , plug-in hybrid vehicle , or plug-in hybrid is a hybrid vehicle which utilizes rechargeable batteries, or another energy storage device, that can be restored to full charge by connecting a plug to an external electric power source...
, or PHEVs, which use a hybrid strategy of electrical and fuel storage for their energy needs.
Hydrogen storage has been proposed by some to be optimal in a narrow range of energy storage time, probably somewhere between a few days and a few weeks. This range is subject to further narrowing with any improvements in battery technology. It is always possible that some kind of breakthrough in hydrogen storage or generation could occur, but this is unlikely given the physical and chemical limitations of the technical choices are fairly well understood.
Captive hydrogen synthetic methane production
In a similar way as with synthetic alcohol production, hydrogen can be used on-site to directly (nonbiologically) produce greenhouse-neutral gaseous fuels. Thus, captive-hydrogen-mediated production of greenhouse-neutral methaneMethane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
has been proposed (note that this is the reverse of the present method of acquiring hydrogen from natural methane, but one that does not require ultimate burning and release of fossil fuel carbon). Captive hydrogen (and carbon dioxide) may be used onsite to synthesize methane, using the Sabatier reaction
Sabatier reaction
The Sabatier reaction or Sabatier process involves the reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst to produce methane and water. Optionally ruthenium on alumina makes a more efficient catalyst...
. This process is about 80% efficient, reducing the round trip efficiency to about 20 to 30%, depending on the method of fuel utilization. This is even lower than hydrogen, but the storage costs drop by at least a factor of 3, because of methane's higher boiling point and higher energy density. Liquid methane has 3.2 times the energy density of liquid hydrogen and is easier to store. Additionally, the pipe infrastructure (natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
pipelines) are already in place. Natural-gas-powered vehicles already exist, and are known to be easier to adapt from existing internal engine technology, than internal combustion autos running directly on hydrogen. Experience with natural gas powered vehicles shows that methane storage is inexpensive, once one has accepted the cost of conversion to store the fuel. However, the cost of alcohol storage is even lower, so this technology would need to produce methane at a considerable savings with regard to alcohol production. Ultimate mature prices of fuels in the competing technologies are not presently known, but both are expected to offer substantial infrastructural savings over attempts to transport and use hydrogen directly.
See also
- Alternative fuelAlternative fuelAlternative fuels, known as non-conventional or advanced fuels, are any materials or substances that can be used as fuels, other than conventional fuels...
- Energy developmentEnergy developmentEnergy development is the effort to provide sufficient primary energy sources and secondary energy forms for supply, cost, impact on air pollution and water pollution, mitigation of climate change with renewable energy....
- Fuel Cells and Hydrogen Joint Technology Initiative
- HOPE Curriculum (Hydrogen Outreach Program for Education)
- Hydrogen energy plant in DenmarkHydrogen energy plant in DenmarkDenmark's first full-scale wind-Hydrogen energy plant and testing facility, the Lolland Hydrogen Community, began operation in May 2007. It is also the European Union's first full-scale Hydrogen Community Demonstration facility for residential Fuel Cell Combined Heat and Power .Located in the city...
- Qazvin hydrogen power plantQazvin hydrogen power plantQazvin hydrogen power plant or Talaqan hydrogen power plant is a pilot hydrogen power plant built by Renewable energies organization of Iran in Qazvin Province, Iran. The work on the plant started in 2001 and it became operational in 2009. The idea of the plant is based on solar–hydrogen energy cycle...
- Hydrogen internal combustion engine vehicleHydrogen internal combustion engine vehicleA hydrogen internal combustion engine vehicle is a type of hydrogen vehicle using an internal combustion engine. Hydrogen internal combustion engine vehicles are different from hydrogen fuel cell vehicles ; the hydrogen internal combustion engine is simply a modified version of the traditional...
- Hydrogen prizeHydrogen prizeThe Hydrogen Prize is a proposed financial award to encourage research into hydrogen as an alternative fuel.-Legislative status:During October 2008, the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy announced that the Hydrogen Education Foundation of Washington, D.C...
- Hydrogen vehicleHydrogen vehicleA hydrogen vehicle is a vehicle that uses hydrogen as its onboard fuel for motive power. Hydrogen vehicles include hydrogen fueled space rockets, as well as automobiles and other transportation vehicles...
- International Centre for Hydrogen Energy TechnologiesInternational Centre for Hydrogen Energy TechnologiesThe International Centre for Hydrogen Energy Technologies . Its role is to support, demonstrate and promote viable implementations of hydrogen energy technologies with the aims of enhancing future economic development, particularly in emerging countries...
- International Journal of Hydrogen EnergyInternational Journal of Hydrogen EnergyThe International Journal of Hydrogen Energy is a peer-reviewed scientific journal published for the International Association for Hydrogen Energy by Elsevier. It covers all aspects of hydrogen generation and storage....
- Lolland Hydrogen Community
Further reading
Author interview at Global Public Media. Hydrogen economy = "laughable a fantasy" p. 115 Summary This book is available online in full text:External links
- Hydrogen Society
- International Partnership for the Hydrogen Economy
- European Hydrogen Association
- European Network of Excellence Safety of Hydrogen as an Energy Carrier (HySafe)
- World's First Higher Educational Programme in Hydrogen Safety Engineering
- Canada
- U.S.-Department of Energy
- [ftp://ftp.cordis.europa.eu/pub/fp7/energy/docs/hydrogen_synopses_en.pdf European Projects 2002-2006 FP6]
- European Projects 2007-2013 FP7
- 20 Hydrogen myths - Published by the Rocky Mountain InstituteRocky Mountain InstituteRocky Mountain Institute is an organization in the United States dedicated to research, publication, consulting, and lecturing in the general field of sustainability, with a special focus on profitable innovations for energy and resource efficiency. RMI was established in 1982 and has grown into a...
, a major hydrogen economy proponent. - Does a Hydrogen Economy Make Sense?
- Hydrogen and Fuel Cell Wiki
- ITM Power - Economic renewable hydrogen from low cost materials (non platinum, fluorocarbon free) & manufacturing processes - electrolyzers & fuel cells
- The Hydrogen Hoax article by Robert Zubrin in The New Atlantis
- Resources on hydrogen plants

