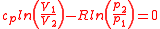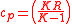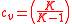Otto cycle
Encyclopedia
An Otto cycle is an idealized thermodynamic cycle
which describes the functioning of a typical reciprocating piston engine, the thermodynamic cycle most commonly found in automobile engines.
The Otto cycle is constructed out of:
The adiabatic processes are impermeable to heat: heat flows into the loop through the left pressurizing process and some of it flows back out through the right depressurizing process, and the heat which remains does the work.
The processes are described by :
The Otto cycle consists of adiabatic
compression, heat addition at constant volume, adiabatic expansion, and rejection of heat at constant volume. In the case of a four-stroke Otto cycle, technically there are two additional processes: one for the exhaust of waste heat and combustion products (by isobaric
compression), and one for the intake of cool oxygen-rich air (by isobaric expansion); however, these are often omitted in a simplified analysis. Even though these two processes are critical to the functioning of a real engine, wherein the details of heat transfer and combustion chemistry are relevant, for the simplified analysis of the thermodynamic cycle, it is simpler and more convenient to assume that all of the waste-heat is removed during a single volume change.
A P-V animation of the Otto cycle is very useful in the analysis of the entire process.
The first person to build a working four stroke engine, a stationary engine using a coal gas-air mixture for fuel (a gas engine
), was German
engineer Nicolaus Otto
. This is why the four-stroke principle today is commonly known as the Otto cycle and four-stroke engines using spark plug
s often are called Otto engines.
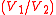 . Mechanically this is the adiabatic compression of the air/fuel mixture in the cylinder, also known as the compression stroke. Generally the compression ratio is around 9-10:1 (V1:V2) for a typical engine.
. Mechanically this is the adiabatic compression of the air/fuel mixture in the cylinder, also known as the compression stroke. Generally the compression ratio is around 9-10:1 (V1:V2) for a typical engine.
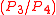 is called the "explosion ratio". At this instant the air/fuel mixture is compressed at the top of the compression stroke with the volume essentially held constant, also known as ignition phase.
is called the "explosion ratio". At this instant the air/fuel mixture is compressed at the top of the compression stroke with the volume essentially held constant, also known as ignition phase.
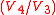 is called "isentropic expansion ratio". Mechanically this is the adiabatic expansion of the hot gaseous mixture in the cylinder head, also known as expansion (power) stroke.
is called "isentropic expansion ratio". Mechanically this is the adiabatic expansion of the hot gaseous mixture in the cylinder head, also known as expansion (power) stroke.
Induction stroke-intake of the next air charge into the cylinder. The volume of the exhaust gasses is the same as the air charge.
is rewritten as:

Applying this to the Otto cycle the four process equations can be derived:
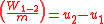
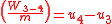
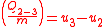
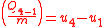
Since the first law is expressed as heat added to the system and work expelled from the system then (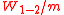 ) and (
) and (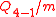 ) will always produce positive values. However, since work always involves movement, processes 2-3 and 4-1 will be omitted because they occur at a constant volume. The net work can be expressed as:
) will always produce positive values. However, since work always involves movement, processes 2-3 and 4-1 will be omitted because they occur at a constant volume. The net work can be expressed as:
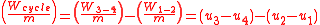
The net work can also be found by evaluating the heat added minus the heat leaving or expelled.
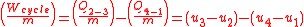
Thermal efficiency
is the quotient of the net work to the heat addition into system. Upon rearrangement the thermal efficiency can be obtained (Net Work/Heat added):
Equation 1: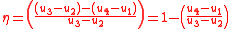
Alternatively, thermal efficiency can be derived by strictly heat added and heat rejected.


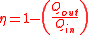
In the Otto cycle, there is no heat transfer during the process 1-2 and 3-4 as they are reversible adiabatic processes. Heat is supplied only during the constant volume processes 2-3 and heat is rejected only during the constant volume processes 4-1.
Equation 1 can now be related to the specific heat equation for constant volume. The specific heats
are particularly useful for thermodynamic calculations involving the ideal gas
model.
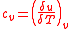
Rearranging yields:
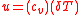
Inserting the specific heat equation into the thermal efficiency equation (Equation 1) yields.
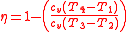
Upon rearrangement:
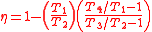
Next, noting from the diagrams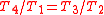 , thus both of these can be omitted. The equation then reduces to:
, thus both of these can be omitted. The equation then reduces to:
Equation 2: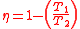
Since the Otto cycle is an isentropic process the isentropic equations
of ideal gases and the constant pressure/volume relations can be used to yield Equations 3 & 4.
Equation 3:
Equation 4: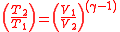
Further simplifying Equation 4, where is the compression ratio
is the compression ratio 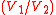 :
:
Equation 5: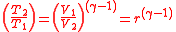
Also, note that
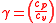
where is the specific heat ratio
is the specific heat ratio
From inverting Equation 4 and inserting it into Equation 2 the final thermal efficiency can be expressed as :
Equation 6:
From analyzing equation 6 it is evident that the Otto cycle depends directly upon the compression ratio . Since the
. Since the  for air is 1.4, an increase in
for air is 1.4, an increase in  will produce an increase in
will produce an increase in 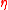 . However, the
. However, the  for the combustion products of the fuel/air mixture is taken at approximately 1.3.
for the combustion products of the fuel/air mixture is taken at approximately 1.3.
The foregoing discussion implies that it is more efficient to have a high compression ratio. The standard ratio is approximately 10:1 for typical automobiles. Usually this does not increase much because of the possibility of autoignition, or "knock
", which places an upper limit on the compression ratio. During the compression process 1-2 the temperature rises, therefore an increase in the compression ratio causes an increase in temperature. Autoignition occurs when the temperature of the fuel/air mixture becomes too high before it is ignited by the flame front. The compression stroke is intended to compress the products before the flame ignites the mixture. Therefore if the compression ratio was increased, the mixture could be compressed before ignition leading to "engine knocking". This can damage engine components and will decrease the original horsepower of the engine.
Thermodynamic cycle
A thermodynamic cycle consists of a series of thermodynamic processes transferring heat and work, while varying pressure, temperature, and other state variables, eventually returning a system to its initial state...
which describes the functioning of a typical reciprocating piston engine, the thermodynamic cycle most commonly found in automobile engines.
The Otto cycle is constructed out of:
- TOP and BOTTOM of the loop: a pair of quasi-parallel adiabatic processAdiabatic processIn thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
es - LEFT and RIGHT sides of the loop: a pair of parallel isochoric processIsochoric processAn isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant...
es
The adiabatic processes are impermeable to heat: heat flows into the loop through the left pressurizing process and some of it flows back out through the right depressurizing process, and the heat which remains does the work.
The processes are described by :
- Process 1-2 is an isentropicIsentropic processIn thermodynamics, an isentropic process or isoentropic process is one in which for purposes of engineering analysis and calculation, one may assume that the process takes place from initiation to completion without an increase or decrease in the entropy of the system, i.e., the entropy of the...
compression of the air as the piston moves from bottom dead center to top dead center. - Process 2-3 is a constant-volume heat transfer to the air from an external source while the piston is at top dead center. This process is intended to represent the ignition of the fuel-air mixture and the subsequent rapid burning.
- Process 3-4 is an isentropic expansion (power stroke).
- Process 4-1 completes the cycle by a constant-volume process in which heat is rejected from the air while the piston is a bottom dead center.
The Otto cycle consists of adiabatic
Adiabatic process
In thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
compression, heat addition at constant volume, adiabatic expansion, and rejection of heat at constant volume. In the case of a four-stroke Otto cycle, technically there are two additional processes: one for the exhaust of waste heat and combustion products (by isobaric
Isobaric process
An isobaric process is a thermodynamic process in which the pressure stays constant. The term derives from the Greek isos, , and barus,...
compression), and one for the intake of cool oxygen-rich air (by isobaric expansion); however, these are often omitted in a simplified analysis. Even though these two processes are critical to the functioning of a real engine, wherein the details of heat transfer and combustion chemistry are relevant, for the simplified analysis of the thermodynamic cycle, it is simpler and more convenient to assume that all of the waste-heat is removed during a single volume change.
A P-V animation of the Otto cycle is very useful in the analysis of the entire process.
History
The four-stroke engine was first patented by Alphonse Beau de Rochas in 1861. Before, in about 1854–57, two Italians (Eugenio Barsanti and Felice Matteucci) invented an engine that was rumored to be very similar, but the patent was lost.The first person to build a working four stroke engine, a stationary engine using a coal gas-air mixture for fuel (a gas engine
Gas engine
A gas engine means an engine running on a gas, such as coal gas, producer gas biogas, landfill gas, or natural gas. In the UK, the term is unambiguous...
), was German
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
engineer Nicolaus Otto
Nicolaus Otto
Nikolaus August Otto was the German inventor of the first internal-combustion engine to efficiently burn fuel directly in a piston chamber. Although other internal combustion engines had been invented these were not based on four separate strokes...
. This is why the four-stroke principle today is commonly known as the Otto cycle and four-stroke engines using spark plug
Spark plug
A spark plug is an electrical device that fits into the cylinder head of some internal combustion engines and ignites compressed fuels such as aerosol, gasoline, ethanol, and liquefied petroleum gas by means of an electric spark.Spark plugs have an insulated central electrode which is connected by...
s often are called Otto engines.
Process 1-2 (B on diagrams)
Piston moves from crank end (bottom dead center BDC) to cover end (top dead center TDC) and an ideal gas with initial state 1 is compressed isentropically to state point 2, through compression ratioCompression ratio
The 'compression ratio' of an internal-combustion engine or external combustion engine is a value that represents the ratio of the volume of its combustion chamber from its largest capacity to its smallest capacity...
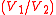 . Mechanically this is the adiabatic compression of the air/fuel mixture in the cylinder, also known as the compression stroke. Generally the compression ratio is around 9-10:1 (V1:V2) for a typical engine.
. Mechanically this is the adiabatic compression of the air/fuel mixture in the cylinder, also known as the compression stroke. Generally the compression ratio is around 9-10:1 (V1:V2) for a typical engine.Process 2-3 (C on diagrams)
The piston is momentarily at rest at TDC and heat is added to the working fluid at constant volume from an external heat source which is brought into contact with the cylinder head. The pressure rises and the ratio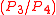 is called the "explosion ratio". At this instant the air/fuel mixture is compressed at the top of the compression stroke with the volume essentially held constant, also known as ignition phase.
is called the "explosion ratio". At this instant the air/fuel mixture is compressed at the top of the compression stroke with the volume essentially held constant, also known as ignition phase.Process 3-4 (D on diagrams)
The increased high pressure exerts a greater amount of force on the piston and pushes it towards the BDC. Expansion of working fluid takes place isentropically and work is done by the system. The volume ratio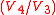 is called "isentropic expansion ratio". Mechanically this is the adiabatic expansion of the hot gaseous mixture in the cylinder head, also known as expansion (power) stroke.
is called "isentropic expansion ratio". Mechanically this is the adiabatic expansion of the hot gaseous mixture in the cylinder head, also known as expansion (power) stroke.Process 4-1 (A on diagrams)
The piston is momentarily at rest at BDC and heat is rejected to the external sink by bringing it in contact with the cylinder head. The process is so controlled that ultimately the working fluid comes to its initial state 1 and the cycle is completed. Many petrol and gas engines work on a cycle which is a slight modification of Otto cycle. This cycle is called "constant volume cycle" because the heat is supplied to air at constant volume.Exhaust and intake strokes
Exhaust stroke-ejection of the gaseous mixture via an exhaust valve through the cylinder head.Induction stroke-intake of the next air charge into the cylinder. The volume of the exhaust gasses is the same as the air charge.
Cycle Analysis
Processes 1-2 and 3-4 do work on the system but no heat transfer occurs during adiabatic expansion and compression. Processes 2-3 and 4-1 are isochoric therefore heat transfer occurs but no work is done. No work is done during a isochoric (constant volume) because work requires movement; when the piston volume does not change no shaft work is produced by the system. Four different equations can be derived by neglecting kinetic and potential energy and considering the first law of thermodynamics (energy conservation). Assuming these conditions the first lawFirst law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
is rewritten as:

Applying this to the Otto cycle the four process equations can be derived:
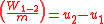
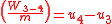
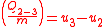
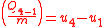
Since the first law is expressed as heat added to the system and work expelled from the system then (
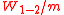 ) and (
) and (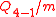 ) will always produce positive values. However, since work always involves movement, processes 2-3 and 4-1 will be omitted because they occur at a constant volume. The net work can be expressed as:
) will always produce positive values. However, since work always involves movement, processes 2-3 and 4-1 will be omitted because they occur at a constant volume. The net work can be expressed as: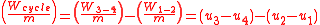
The net work can also be found by evaluating the heat added minus the heat leaving or expelled.
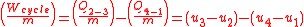
Thermal efficiency
Thermal efficiency
In thermodynamics, the thermal efficiency is a dimensionless performance measure of a device that uses thermal energy, such as an internal combustion engine, a boiler, a furnace, or a refrigerator for example.-Overview:...
is the quotient of the net work to the heat addition into system. Upon rearrangement the thermal efficiency can be obtained (Net Work/Heat added):
Equation 1:
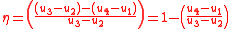
Alternatively, thermal efficiency can be derived by strictly heat added and heat rejected.


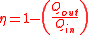
In the Otto cycle, there is no heat transfer during the process 1-2 and 3-4 as they are reversible adiabatic processes. Heat is supplied only during the constant volume processes 2-3 and heat is rejected only during the constant volume processes 4-1.
Equation 1 can now be related to the specific heat equation for constant volume. The specific heats
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
are particularly useful for thermodynamic calculations involving the ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
model.
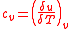
Rearranging yields:
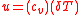
Inserting the specific heat equation into the thermal efficiency equation (Equation 1) yields.
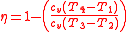
Upon rearrangement:
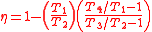
Next, noting from the diagrams
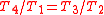 , thus both of these can be omitted. The equation then reduces to:
, thus both of these can be omitted. The equation then reduces to:Equation 2:
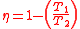
Since the Otto cycle is an isentropic process the isentropic equations
Isentropic process
In thermodynamics, an isentropic process or isoentropic process is one in which for purposes of engineering analysis and calculation, one may assume that the process takes place from initiation to completion without an increase or decrease in the entropy of the system, i.e., the entropy of the...
of ideal gases and the constant pressure/volume relations can be used to yield Equations 3 & 4.
Equation 3:

Equation 4:
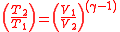
-
-
-
- The derivation of the previous equations are found by solving these four equations respectively (where
 is the gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
is the gas constantGas constantThe gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
):
- The derivation of the previous equations are found by solving these four equations respectively (where
-
-
Further simplifying Equation 4, where
 is the compression ratio
is the compression ratio 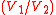 :
:Equation 5:
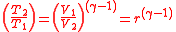
Also, note that
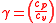
where
 is the specific heat ratio
is the specific heat ratioFrom inverting Equation 4 and inserting it into Equation 2 the final thermal efficiency can be expressed as :
Equation 6:

From analyzing equation 6 it is evident that the Otto cycle depends directly upon the compression ratio
 . Since the
. Since the  for air is 1.4, an increase in
for air is 1.4, an increase in  will produce an increase in
will produce an increase in 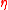 . However, the
. However, the  for the combustion products of the fuel/air mixture is taken at approximately 1.3.
for the combustion products of the fuel/air mixture is taken at approximately 1.3.The foregoing discussion implies that it is more efficient to have a high compression ratio. The standard ratio is approximately 10:1 for typical automobiles. Usually this does not increase much because of the possibility of autoignition, or "knock
Engine knocking
Knocking in spark-ignition internal combustion engines occurs when combustion of the air/fuel mixture in the cylinder starts off correctly in response to ignition by the spark plug, but one or more pockets of air/fuel mixture explode outside the envelope of the normal combustion front.The...
", which places an upper limit on the compression ratio. During the compression process 1-2 the temperature rises, therefore an increase in the compression ratio causes an increase in temperature. Autoignition occurs when the temperature of the fuel/air mixture becomes too high before it is ignited by the flame front. The compression stroke is intended to compress the products before the flame ignites the mixture. Therefore if the compression ratio was increased, the mixture could be compressed before ignition leading to "engine knocking". This can damage engine components and will decrease the original horsepower of the engine.