
Thermal efficiency
Encyclopedia
In thermodynamics
, the thermal efficiency (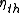 ) is a dimensionless
) is a dimensionless
performance measure of a device that uses thermal energy
, such as an internal combustion engine
, a boiler
, a furnace
, or a refrigerator
for example.
is the ratio
between the useful output of a device and the input, in energy
terms. For thermal efficiency, the input,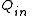 , to the device is heat
, to the device is heat
, or the heat-content of a fuel that is consumed. The desired output is mechanical work
,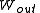 , or heat,
, or heat, 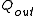 , or possibly both. Because the input heat normally has a real financial cost, a memorable, generic definition of thermal efficiency is
, or possibly both. Because the input heat normally has a real financial cost, a memorable, generic definition of thermal efficiency is
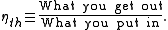
From the first law of thermodynamics
, the energy output cannot exceed the input, so
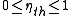
When expressed as a percentage, the thermal efficiency must be between 0% and 100%. Due to inefficiencies such as friction, heat loss, and other factors, thermal engines' efficiencies are typically much less than 100%. For example, a typical gasoline automobile engine operates at around 25% efficiency, and a large coal-fueled electrical generating plant peaks at about 46%. The largest diesel engine in the world
peaks at 51.7%. In a combined cycle
plant, thermal efficiencies are approaching 60%. Such a real-world value may be used as a figure of merit
for the device.
There are two types of thermal efficiency: indicated thermal efficiency and brake thermal efficiency.
, or heat, Qin into mechanical energy
, or work
, Wout. They cannot do this task perfectly, so some of the input heat energy is not converted into work, but is dissipated as waste heat
Qout into the environment
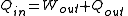
The thermal efficiency of a heat engine
is the percentage of heat energy that is transformed into work
. Thermal efficiency is defined as
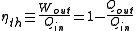
The efficiency of even the best heat engines is low; usually below 50% and often far below. So the energy lost to the environment by heat engines is a major waste of energy resources, although modern cogeneration
, combined cycle
and energy recycling
schemes are beginning to use this heat for other purposes. Since a large fraction of the fuels produced worldwide go to powering heat engines, perhaps up to half of the useful energy produced worldwide is wasted in engine inefficiency. This inefficiency can be attributed to three causes. There is an overall theoretical limit to the efficiency of any heat engine due to temperature, called the Carnot efficiency. Second, specific types of engines have lower limits on their efficiency due to the inherent irreversibility
of the engine cycle they use. Thirdly, the nonideal behavior of real engines, such as mechanical friction
and losses in the combustion
process causes further efficiency losses.
puts a fundamental limit on the thermal efficiency of all heat engines. Surprisingly, even an ideal, frictionless engine can't convert anywhere near 100% of its input heat into work. The limiting factors are the temperature at which the heat enters the engine,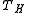 , and the temperature of the environment into which the engine exhausts its waste heat,
, and the temperature of the environment into which the engine exhausts its waste heat, 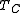 , measured in an absolute scale, such as the Kelvin
, measured in an absolute scale, such as the Kelvin
or Rankine
scale. From Carnot's theorem, for any engine working between these two temperatures:
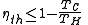
This limiting value is called the Carnot cycle efficiency because it is the efficiency of an unattainable, ideal, reversible
engine cycle called the Carnot cycle
. No device converting heat into mechanical energy, regardless of its construction, can exceed this efficiency.
Examples of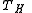 are the temperature of hot steam entering the turbine of a steam power plant, or the temperature at which the fuel burns in an internal combustion engine
are the temperature of hot steam entering the turbine of a steam power plant, or the temperature at which the fuel burns in an internal combustion engine
.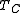 is usually the ambient temperature where the engine is located, or the temperature of a lake or river that waste heat is discharged into. For example, if an automobile engine burns gasoline at a temperature of
is usually the ambient temperature where the engine is located, or the temperature of a lake or river that waste heat is discharged into. For example, if an automobile engine burns gasoline at a temperature of 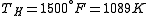 and the ambient temperature is
and the ambient temperature is 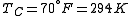 , then its maximum possible efficiency is:
, then its maximum possible efficiency is:
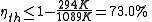
Due to the other causes detailed below, practical engines have efficiencies far below the Carnot limit; for example the average automobile engine is less than 35% efficient.
As Carnot's theorem only applies to heat engines, devices that convert the fuel's energy directly into work without burning it, such as fuel cell
s, can exceed the Carnot efficiency.
It can be seen that since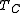 is fixed by the environment, the only way for a designer to increase the Carnot efficiency of an engine is to increase
is fixed by the environment, the only way for a designer to increase the Carnot efficiency of an engine is to increase 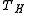 , the temperature at which the heat is added to the engine. This is a general principle that applies to all heat engines: the efficiency increases with operating temperature. For this reason the operating temperatures of engines have increased greatly over the long term, and new materials such as ceramics to enable engines to stand higher temperatures are an active area of research.
, the temperature at which the heat is added to the engine. This is a general principle that applies to all heat engines: the efficiency increases with operating temperature. For this reason the operating temperatures of engines have increased greatly over the long term, and new materials such as ceramics to enable engines to stand higher temperatures are an active area of research.
and thus represents the upper limit on efficiency of an engine cycle. Practical engine cycles are irreversible and thus have inherently lower efficiency than the Carnot efficiency when operated between the same temperatures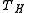 and
and 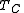 . One of the factors determining efficiency is how heat is added to the working fluid in the cycle, and how it is removed. The Carnot cycle achieves maximum efficiency because all the heat is added to the working fluid at the maximum temperature
. One of the factors determining efficiency is how heat is added to the working fluid in the cycle, and how it is removed. The Carnot cycle achieves maximum efficiency because all the heat is added to the working fluid at the maximum temperature 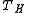 , and removed at the minimum temperature
, and removed at the minimum temperature 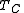 . In contrast, in an internal combustion engine, the temperature of the fuel-air mixture in the cylinder is nowhere near its peak temperature as the fuel starts to burn, and only reaches the peak temperature as all the fuel is consumed, so the average temperature at which heat is added is lower, reducing efficiency.
. In contrast, in an internal combustion engine, the temperature of the fuel-air mixture in the cylinder is nowhere near its peak temperature as the fuel starts to burn, and only reaches the peak temperature as all the fuel is consumed, so the average temperature at which heat is added is lower, reducing efficiency.
. Real engines have many departures from ideal behavior that waste energy, reducing actual efficiencies far below the theoretical values given above. Examples are:
Another source of inefficiency is that engines must be optimized for other goals besides efficiency, such as low pollution
. The requirements for vehicle engines are particularly stringent: they must be designed for low emissions, adequate acceleration, fast starting, light weight, low noise, etc. These require compromises in design (such as altered valve timing
to reduce emissions) that reduce efficiency. The average automobile engine is only about 35% efficient, and must also be kept idling at stoplights, wasting an additional 17% of the energy, resulting in an overall efficiency of 18%. Large stationary electric generating plants
have fewer of these competing requirements as well as more efficient Rankine cycles, so they are significantly more efficient than vehicle engines, around 50% Therefore, replacing internal combustion vehicles with electric vehicle
s, which run on a battery
that is charged with electricity generated by burning fuel in a power plant, has the theoretical potential to increase the thermal efficiency of energy use in transportation, thus decreasing the demand for fossil fuels.
When comparing different heat engines as sources of power, such as electric power or the power to run vehicles, the engine efficiency alone is only one factor. To give a meaningful comparison, the overall efficiency of the entire energy supply chain from the fuel source to the consumer must be considered. Although the heat wasted by heat engines is usually the largest source of inefficiency, factors such as the energy cost of fuel refining and transportation, and energy loss in electrical transmission lines to transport it, may offset the advantage of a more efficient heat engine.
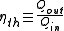
where the quantities are heat-equivalent values.
quantities are heat-equivalent values.
So, for a boiler that produces 210 kW (or 700,000 BTU/h) output for each 300 kW (or 1,000,000 BTU/h) heat-equivalent input, its thermal efficiency is 210/300 = 0.70, or 70%. This means that 30% of the energy is lost to the environment.
An electric resistance heater has a thermal efficiency close to 100%. When comparing heating units, such as a highly efficient electric resistance heater to an 80% efficient natural gas-fueled furnace, an economic analysis is needed to determine the most cost-effective choice.
is the amount of heat
released during an exothermic reaction
(e.g., combustion
) and is a characteristic of each substance. It is measured in units of energy
per unit of the substance, usually mass
, such as: kcal
/kg, kJ/kg, J
/mol
, Btu
/m³.
The heating value for fuel
s is expressed as the HHV, LHV, or GHV to distinguish treatment of the heat of phase changes:
Which definition of heating value is being used significantly affects any quoted efficiency. Not stating whether an efficiency is HHV or LHV renders such numbers very misleading.
s, refrigerator
s and air conditioners use work to move heat from a colder to a warmer place, so their function is the opposite of a heat engine. The work energy (Win) that is applied to them is converted into heat, and the sum of this energy and the heat energy that is moved from the cold reservoir (QC) is equal to the total heat energy added to the hot reservoir (QH)
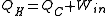
Their efficiency is measured by a coefficient of performance (COP). Heat pumps are measured by the efficiency with which they add heat to the hot reservoir, COPheating; refrigerators and air conditioners by the efficiency with which they remove heat from the cold interior, COPcooling:
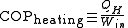
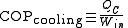
The reason for not using the term 'efficiency' is that the coefficient of performance can often be greater than 100%. Since these devices are moving heat, not creating it, the amount of heat they move can be greater than the input work. Therefore, heat pumps can be a more efficient way of heating than simply converting the input work into heat, as in an electric heater or furnace.
Since they are heat engines, these devices are also limited by Carnot's theorem. The limiting value of the Carnot 'efficiency' for these processes, with the equality theoretically achievable only with an ideal 'reversible' cycle, is:
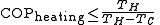
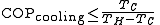
The same device used between the same temperatures is more efficient when considered as a heat pump than when considered as a refrigerator:
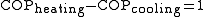
This is because when heating, the work used to run the device is converted to heat and adds to the desired effect, whereas if the desired effect is cooling the heat resulting from the input work is just an unwanted byproduct.
is the more common measure of energy efficiency for cooling devices, as well as for heat pumps when in their heating mode. For energy-conversion heating devices their peak steady-state thermal efficiency is often stated, e.g., 'this furnace is 90% efficient', but a more detailed measure of seasonal energy effectiveness is the Annual Fuel Utilization Efficiency
(AFUE).
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, the thermal efficiency (
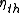 ) is a dimensionless
) is a dimensionlessDimensionless quantity
In dimensional analysis, a dimensionless quantity or quantity of dimension one is a quantity without an associated physical dimension. It is thus a "pure" number, and as such always has a dimension of 1. Dimensionless quantities are widely used in mathematics, physics, engineering, economics, and...
performance measure of a device that uses thermal energy
Thermal energy
Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature....
, such as an internal combustion engine
Internal combustion engine
The internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine...
, a boiler
Boiler
A boiler is a closed vessel in which water or other fluid is heated. The heated or vaporized fluid exits the boiler for use in various processes or heating applications.-Materials:...
, a furnace
Furnace
A furnace is a device used for heating. The name derives from Latin fornax, oven.In American English and Canadian English, the term furnace on its own is generally used to describe household heating systems based on a central furnace , and sometimes as a synonym for kiln, a device used in the...
, or a refrigerator
Refrigerator
A refrigerator is a common household appliance that consists of a thermally insulated compartment and a heat pump that transfers heat from the inside of the fridge to its external environment so that the inside of the fridge is cooled to a temperature below the ambient temperature of the room...
for example.
Overview
In general, energy conversion efficiencyEnergy conversion efficiency
Energy conversion efficiency is the ratio between the useful output of an energy conversion machine and the input, in energy terms. The useful output may be electric power, mechanical work, or heat.-Overview:...
is the ratio
Ratio
In mathematics, a ratio is a relationship between two numbers of the same kind , usually expressed as "a to b" or a:b, sometimes expressed arithmetically as a dimensionless quotient of the two which explicitly indicates how many times the first number contains the second In mathematics, a ratio is...
between the useful output of a device and the input, in energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
terms. For thermal efficiency, the input,
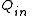 , to the device is heat
, to the device is heatHeat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
, or the heat-content of a fuel that is consumed. The desired output is mechanical work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
,
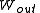 , or heat,
, or heat, 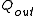 , or possibly both. Because the input heat normally has a real financial cost, a memorable, generic definition of thermal efficiency is
, or possibly both. Because the input heat normally has a real financial cost, a memorable, generic definition of thermal efficiency is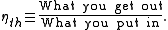
From the first law of thermodynamics
First law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
, the energy output cannot exceed the input, so
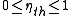
When expressed as a percentage, the thermal efficiency must be between 0% and 100%. Due to inefficiencies such as friction, heat loss, and other factors, thermal engines' efficiencies are typically much less than 100%. For example, a typical gasoline automobile engine operates at around 25% efficiency, and a large coal-fueled electrical generating plant peaks at about 46%. The largest diesel engine in the world
Wärtsilä-Sulzer RTA96-C
The Wärtsilä RT-flex96C is a two-stroke turbocharged low-speed diesel engine designed by the Finnish manufacturer Wärtsilä. It is currently considered the largest reciprocating engine in the world, designed for large container ships, running on heavy fuel oil...
peaks at 51.7%. In a combined cycle
Combined cycle
In electric power generation a combined cycle is an assembly of heat engines that work in tandem off the same source of heat, converting it into mechanical energy, which in turn usually drives electrical generators...
plant, thermal efficiencies are approaching 60%. Such a real-world value may be used as a figure of merit
Figure of merit
A figure of merit is a quantity used to characterize the performance of a device, system or method, relative to its alternatives. In engineering, figures of merit are often defined for particular materials or devices in order to determine their relative utility for an application...
for the device.
There are two types of thermal efficiency: indicated thermal efficiency and brake thermal efficiency.
Heat engines
Heat engines transform thermal energyThermal energy
Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature....
, or heat, Qin into mechanical energy
Mechanical energy
In physics, mechanical energy is the sum of potential energy and kinetic energy present in the components of a mechanical system. It is the energy associated with the motion and position of an object. The law of conservation of energy states that in an isolated system that is only subject to...
, or work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
, Wout. They cannot do this task perfectly, so some of the input heat energy is not converted into work, but is dissipated as waste heat
Waste heat
Waste heat sometimes called Secondary heat or Low-grade heat refers to heat produced by machines, electrical equipment and industrial processes for which no useful application is found. Energy is often produced by a heat engine, running on a source of high-temperature heat...
Qout into the environment
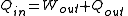
The thermal efficiency of a heat engine
Heat engine
In thermodynamics, a heat engine is a system that performs the conversion of heat or thermal energy to mechanical work. It does this by bringing a working substance from a high temperature state to a lower temperature state. A heat "source" generates thermal energy that brings the working substance...
is the percentage of heat energy that is transformed into work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
. Thermal efficiency is defined as
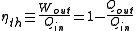
The efficiency of even the best heat engines is low; usually below 50% and often far below. So the energy lost to the environment by heat engines is a major waste of energy resources, although modern cogeneration
Cogeneration
Cogeneration is the use of a heat engine or a power station to simultaneously generate both electricity and useful heat....
, combined cycle
Combined cycle
In electric power generation a combined cycle is an assembly of heat engines that work in tandem off the same source of heat, converting it into mechanical energy, which in turn usually drives electrical generators...
and energy recycling
Energy recycling
Energy recycling is the energy recovery process of utilizing energy that would normally be wasted, usually by converting it into electricity or thermal energy. Undertaken at manufacturing facilities, power plants, and large institutions such as hospitals and universities, it significantly...
schemes are beginning to use this heat for other purposes. Since a large fraction of the fuels produced worldwide go to powering heat engines, perhaps up to half of the useful energy produced worldwide is wasted in engine inefficiency. This inefficiency can be attributed to three causes. There is an overall theoretical limit to the efficiency of any heat engine due to temperature, called the Carnot efficiency. Second, specific types of engines have lower limits on their efficiency due to the inherent irreversibility
Irreversibility
In science, a process that is not reversible is called irreversible. This concept arises most frequently in thermodynamics, as applied to processes....
of the engine cycle they use. Thirdly, the nonideal behavior of real engines, such as mechanical friction
Friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and/or material elements sliding against each other. There are several types of friction:...
and losses in the combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
process causes further efficiency losses.
Carnot efficiency
The second law of thermodynamicsSecond law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
puts a fundamental limit on the thermal efficiency of all heat engines. Surprisingly, even an ideal, frictionless engine can't convert anywhere near 100% of its input heat into work. The limiting factors are the temperature at which the heat enters the engine,
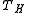 , and the temperature of the environment into which the engine exhausts its waste heat,
, and the temperature of the environment into which the engine exhausts its waste heat, 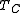 , measured in an absolute scale, such as the Kelvin
, measured in an absolute scale, such as the KelvinKelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
or Rankine
Rankine
Rankine is a thermodynamic temperature scale named after the Scottish engineer and physicist William John Macquorn Rankine, who proposed it in 1859....
scale. From Carnot's theorem, for any engine working between these two temperatures:
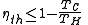
This limiting value is called the Carnot cycle efficiency because it is the efficiency of an unattainable, ideal, reversible
Reversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
engine cycle called the Carnot cycle
Carnot cycle
The Carnot cycle is a theoretical thermodynamic cycle proposed by Nicolas Léonard Sadi Carnot in 1824 and expanded by Benoit Paul Émile Clapeyron in the 1830s and 40s. It can be shown that it is the most efficient cycle for converting a given amount of thermal energy into work, or conversely,...
. No device converting heat into mechanical energy, regardless of its construction, can exceed this efficiency.
Examples of
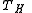 are the temperature of hot steam entering the turbine of a steam power plant, or the temperature at which the fuel burns in an internal combustion engine
are the temperature of hot steam entering the turbine of a steam power plant, or the temperature at which the fuel burns in an internal combustion engineInternal combustion engine
The internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine...
.
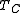 is usually the ambient temperature where the engine is located, or the temperature of a lake or river that waste heat is discharged into. For example, if an automobile engine burns gasoline at a temperature of
is usually the ambient temperature where the engine is located, or the temperature of a lake or river that waste heat is discharged into. For example, if an automobile engine burns gasoline at a temperature of 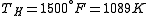 and the ambient temperature is
and the ambient temperature is 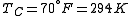 , then its maximum possible efficiency is:
, then its maximum possible efficiency is: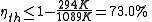
Due to the other causes detailed below, practical engines have efficiencies far below the Carnot limit; for example the average automobile engine is less than 35% efficient.
As Carnot's theorem only applies to heat engines, devices that convert the fuel's energy directly into work without burning it, such as fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
s, can exceed the Carnot efficiency.
It can be seen that since
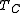 is fixed by the environment, the only way for a designer to increase the Carnot efficiency of an engine is to increase
is fixed by the environment, the only way for a designer to increase the Carnot efficiency of an engine is to increase 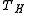 , the temperature at which the heat is added to the engine. This is a general principle that applies to all heat engines: the efficiency increases with operating temperature. For this reason the operating temperatures of engines have increased greatly over the long term, and new materials such as ceramics to enable engines to stand higher temperatures are an active area of research.
, the temperature at which the heat is added to the engine. This is a general principle that applies to all heat engines: the efficiency increases with operating temperature. For this reason the operating temperatures of engines have increased greatly over the long term, and new materials such as ceramics to enable engines to stand higher temperatures are an active area of research.Engine cycle efficiency
The Carnot cycle is reversibleReversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
and thus represents the upper limit on efficiency of an engine cycle. Practical engine cycles are irreversible and thus have inherently lower efficiency than the Carnot efficiency when operated between the same temperatures
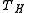 and
and 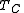 . One of the factors determining efficiency is how heat is added to the working fluid in the cycle, and how it is removed. The Carnot cycle achieves maximum efficiency because all the heat is added to the working fluid at the maximum temperature
. One of the factors determining efficiency is how heat is added to the working fluid in the cycle, and how it is removed. The Carnot cycle achieves maximum efficiency because all the heat is added to the working fluid at the maximum temperature 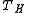 , and removed at the minimum temperature
, and removed at the minimum temperature 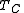 . In contrast, in an internal combustion engine, the temperature of the fuel-air mixture in the cylinder is nowhere near its peak temperature as the fuel starts to burn, and only reaches the peak temperature as all the fuel is consumed, so the average temperature at which heat is added is lower, reducing efficiency.
. In contrast, in an internal combustion engine, the temperature of the fuel-air mixture in the cylinder is nowhere near its peak temperature as the fuel starts to burn, and only reaches the peak temperature as all the fuel is consumed, so the average temperature at which heat is added is lower, reducing efficiency.
- Automobiles: Otto cycle The Otto cycleOtto cycleAn Otto cycle is an idealized thermodynamic cycle which describes the functioning of a typical reciprocating piston engine, the thermodynamic cycle most commonly found in automobile engines....
is the name for the cycle used in spark-ignition internal combustion engineInternal combustion engineThe internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine...
s such as gasoline and hydrogen fuelHydrogen fuelAn ecologically-friendly fuel which uses electrochemical cells or combusts in internal engines to power vehicles and electric devices. It is also used in the propulsion of spacecraft and can potentially be mass produced and commercialized for passenger vehicles and aircraft.In a flame of pure...
ed automobile engines. Its theoretical efficiency depends on the compression ratioCompression ratioThe 'compression ratio' of an internal-combustion engine or external combustion engine is a value that represents the ratio of the volume of its combustion chamber from its largest capacity to its smallest capacity...
r of the engine and the specific heat ratio γ of the gas in the combustion chamber.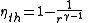
- Hence, to improve efficiency, increase the compression ratio. However the compression ratio is limited by the need to prevent the uncontrolled combustion known as knockingEngine knockingKnocking in spark-ignition internal combustion engines occurs when combustion of the air/fuel mixture in the cylinder starts off correctly in response to ignition by the spark plug, but one or more pockets of air/fuel mixture explode outside the envelope of the normal combustion front.The...
. The specific heat ratio of the air-fuel mixture γ varies somewhat with the fuel, but is generally close to the air value of 1.4. This standard value is usually used in all the engine cycle equations below, and when this approximation is used the cycle is called an air-standard cycle.- Trucks: Diesel cycle In the Diesel cycleDiesel cycleThe Diesel cycle is the thermodynamic cycle which approximates the pressure and volume of the combustion chamber of the Diesel engine, invented by Rudolph Diesel in 1897. It is assumed to have constant pressure during the first part of the "combustion" phase...
used in diesel truck and train enginesDiesel engineA diesel engine is an internal combustion engine that uses the heat of compression to initiate ignition to burn the fuel, which is injected into the combustion chamber...
, the fuel is ignited by compression in the cylinder. The efficiency of the Diesel cycle is dependent on r and γ like the Otto cycle, and also by the cutoff ratio, rc, which is the ratio of the cylinder volume at the beginning and end of the combustion process: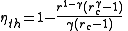
- Trucks: Diesel cycle In the Diesel cycle
- The Diesel cycle is less efficient than the Otto cycle when using the same compression ratio. However, practical Diesel engines are 30% - 35% more efficient than gasoline engines. This is because, since the fuel is not introduced to the combustion chamber until it is required for ignition, the compression ratio is not limited by the need to avoid knocking, so higher ratios are used than in spark ignition engines.
- Power plants: Rankine cycle The Rankine cycleRankine cycleThe Rankine cycle is a cycle that converts heat into work. The heat is supplied externally to a closed loop, which usually uses water. This cycle generates about 90% of all electric power used throughout the world, including virtually all solar thermal, biomass, coal and nuclear power plants. It is...
is the cycle used in steam turbine power plants. The overwhelming majority of the world's electric power is produced with this cycle. Since the cycle's working fluid, water, changes from liquid to vapor and back during the cycle, their efficiencies depend on the thermodynamic properties of water. The thermal efficiency of modern steam turbine plants with reheat cycles can reach 47%, and in combined cycleCombined cycleIn electric power generation a combined cycle is an assembly of heat engines that work in tandem off the same source of heat, converting it into mechanical energy, which in turn usually drives electrical generators...
plants it can approach 60%. - Gas turbines: Brayton cycle The Brayton cycleBrayton cycleThe Brayton cycle is a thermodynamic cycle that describes the workings of the gas turbine engine, basis of the airbreathing jet engine and others. It is named after George Brayton , the American engineer who developed it, although it was originally proposed and patented by Englishman John Barber...
is the cycle used in gas turbineGas turbineA gas turbine, also called a combustion turbine, is a type of internal combustion engine. It has an upstream rotating compressor coupled to a downstream turbine, and a combustion chamber in-between....
s and jet engineJet engineA jet engine is a reaction engine that discharges a fast moving jet to generate thrust by jet propulsion and in accordance with Newton's laws of motion. This broad definition of jet engines includes turbojets, turbofans, rockets, ramjets, pulse jets...
s. It consists of a compressor that increases pressure of the incoming air, then fuel is continuously added to the flow and burned, and the hot exhaust gasses are expanded in a turbine. The efficiency depends largely on the ratio of the pressure inside the combustion chamber p2 to the pressure outside p1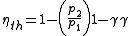
- Power plants: Rankine cycle The Rankine cycle
Other inefficiencies
The above efficiency formulas are based on simple idealized mathematical models of engines, with no friction and working fluids that obey simple thermodynamic rules called the ideal gas lawIdeal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
. Real engines have many departures from ideal behavior that waste energy, reducing actual efficiencies far below the theoretical values given above. Examples are:
- frictionFrictionFriction is the force resisting the relative motion of solid surfaces, fluid layers, and/or material elements sliding against each other. There are several types of friction:...
of moving parts - inefficient combustion
- heat loss from the combustion chamber
- departure of the working fluid from the thermodynamic properties of an ideal gasIdeal gasAn ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
- aerodynamic drag of air moving through the engine
- energy used by auxiliary equipment like oil and water pumps
- inefficient compressors and turbines
- imperfect valve timing
Another source of inefficiency is that engines must be optimized for other goals besides efficiency, such as low pollution
Pollution
Pollution is the introduction of contaminants into a natural environment that causes instability, disorder, harm or discomfort to the ecosystem i.e. physical systems or living organisms. Pollution can take the form of chemical substances or energy, such as noise, heat or light...
. The requirements for vehicle engines are particularly stringent: they must be designed for low emissions, adequate acceleration, fast starting, light weight, low noise, etc. These require compromises in design (such as altered valve timing
Valve timing
In a piston engine, the valve timing is the precise timing of the opening and closing of the valves.In four-stroke cycle engines and some two-stroke cycle engines, the valve timing is controlled by the camshaft. It can be varied by modifying the camshaft, or it can be varied during engine operation...
to reduce emissions) that reduce efficiency. The average automobile engine is only about 35% efficient, and must also be kept idling at stoplights, wasting an additional 17% of the energy, resulting in an overall efficiency of 18%. Large stationary electric generating plants
Electricity generation
Electricity generation is the process of generating electric energy from other forms of energy.The fundamental principles of electricity generation were discovered during the 1820s and early 1830s by the British scientist Michael Faraday...
have fewer of these competing requirements as well as more efficient Rankine cycles, so they are significantly more efficient than vehicle engines, around 50% Therefore, replacing internal combustion vehicles with electric vehicle
Electric vehicle
An electric vehicle , also referred to as an electric drive vehicle, uses one or more electric motors or traction motors for propulsion...
s, which run on a battery
Battery (electricity)
An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy. Since the invention of the first battery in 1800 by Alessandro Volta and especially since the technically improved Daniell cell in 1836, batteries have become a common power...
that is charged with electricity generated by burning fuel in a power plant, has the theoretical potential to increase the thermal efficiency of energy use in transportation, thus decreasing the demand for fossil fuels.
When comparing different heat engines as sources of power, such as electric power or the power to run vehicles, the engine efficiency alone is only one factor. To give a meaningful comparison, the overall efficiency of the entire energy supply chain from the fuel source to the consumer must be considered. Although the heat wasted by heat engines is usually the largest source of inefficiency, factors such as the energy cost of fuel refining and transportation, and energy loss in electrical transmission lines to transport it, may offset the advantage of a more efficient heat engine.
Energy conversion
For a device that converts energy from a form other than thermal energy to another form (such as a boiler or furnace), the thermal efficiency is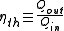
where the
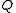 quantities are heat-equivalent values.
quantities are heat-equivalent values.So, for a boiler that produces 210 kW (or 700,000 BTU/h) output for each 300 kW (or 1,000,000 BTU/h) heat-equivalent input, its thermal efficiency is 210/300 = 0.70, or 70%. This means that 30% of the energy is lost to the environment.
An electric resistance heater has a thermal efficiency close to 100%. When comparing heating units, such as a highly efficient electric resistance heater to an 80% efficient natural gas-fueled furnace, an economic analysis is needed to determine the most cost-effective choice.
Effects of fuel heating value
The heating value of a fuelFuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
is the amount of heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
released during an exothermic reaction
Exothermic reaction
An exothermic reaction is a chemical reaction that releases energy in the form of light or heat. It is the opposite of an endothermic reaction. Expressed in a chemical equation:-Overview:...
(e.g., combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
) and is a characteristic of each substance. It is measured in units of energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
per unit of the substance, usually mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
, such as: kcal
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
/kg, kJ/kg, J
Joule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
, Btu
British thermal unit
The British thermal unit is a traditional unit of energy equal to about 1055 joules. It is approximately the amount of energy needed to heat of water, which is exactly one tenth of a UK gallon or about 0.1198 US gallons, from 39°F to 40°F...
/m³.
The heating value for fuel
Fuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
s is expressed as the HHV, LHV, or GHV to distinguish treatment of the heat of phase changes:
- Higher heating value (HHV) is determined by bringing all the products of combustion back to the original pre-combustion temperature, and in particular condensing any vapor produced. This is the same as the thermodynamic heat of combustionHeat of combustionThe heat of combustion is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon reacting with oxygen to form carbon dioxide, water and heat...
. - Lower heating value (LHV) (or net calorific value) is determined by subtracting the heat of vaporization of the water vapor from the higher heating value. The energy required to vaporize the water therefore is not realized as heat.
- Gross heating value accounts for water in the exhaust leaving as vapor, and includes liquid water in the fuel prior to combustion. This value is important for fuels like woodWoodWood is a hard, fibrous tissue found in many trees. It has been used for hundreds of thousands of years for both fuel and as a construction material. It is an organic material, a natural composite of cellulose fibers embedded in a matrix of lignin which resists compression...
or coalCoalCoal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
, which will usually contain some amount of water prior to burning.
Which definition of heating value is being used significantly affects any quoted efficiency. Not stating whether an efficiency is HHV or LHV renders such numbers very misleading.
Heat pumps and refrigerators
Heat pumpHeat pump
A heat pump is a machine or device that effectively "moves" thermal energy from one location called the "source," which is at a lower temperature, to another location called the "sink" or "heat sink", which is at a higher temperature. An air conditioner is a particular type of heat pump, but the...
s, refrigerator
Refrigerator
A refrigerator is a common household appliance that consists of a thermally insulated compartment and a heat pump that transfers heat from the inside of the fridge to its external environment so that the inside of the fridge is cooled to a temperature below the ambient temperature of the room...
s and air conditioners use work to move heat from a colder to a warmer place, so their function is the opposite of a heat engine. The work energy (Win) that is applied to them is converted into heat, and the sum of this energy and the heat energy that is moved from the cold reservoir (QC) is equal to the total heat energy added to the hot reservoir (QH)
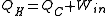
Their efficiency is measured by a coefficient of performance (COP). Heat pumps are measured by the efficiency with which they add heat to the hot reservoir, COPheating; refrigerators and air conditioners by the efficiency with which they remove heat from the cold interior, COPcooling:
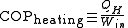
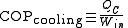
The reason for not using the term 'efficiency' is that the coefficient of performance can often be greater than 100%. Since these devices are moving heat, not creating it, the amount of heat they move can be greater than the input work. Therefore, heat pumps can be a more efficient way of heating than simply converting the input work into heat, as in an electric heater or furnace.
Since they are heat engines, these devices are also limited by Carnot's theorem. The limiting value of the Carnot 'efficiency' for these processes, with the equality theoretically achievable only with an ideal 'reversible' cycle, is:
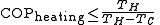
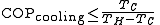
The same device used between the same temperatures is more efficient when considered as a heat pump than when considered as a refrigerator:
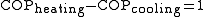
This is because when heating, the work used to run the device is converted to heat and adds to the desired effect, whereas if the desired effect is cooling the heat resulting from the input work is just an unwanted byproduct.
Energy efficiency
The 'thermal efficiency' is sometimes called the energy efficiency. In the United States, in everyday usage the SEERSeasonal energy efficiency ratio
The efficiency of air conditioners is often rated by the Seasonal Energy Efficiency Ratio which is defined by the Air Conditioning, Heating and Refrigeration Institute in its standard ARI 210/240, Performance Rating of Unitary Air-Conditioning and Air-Source Heat Pump Equipment.The SEER rating of...
is the more common measure of energy efficiency for cooling devices, as well as for heat pumps when in their heating mode. For energy-conversion heating devices their peak steady-state thermal efficiency is often stated, e.g., 'this furnace is 90% efficient', but a more detailed measure of seasonal energy effectiveness is the Annual Fuel Utilization Efficiency
Annual fuel utilization efficiency
The annual fuel utilization efficiency is a thermal efficiency measure of combustion equipment like furnaces, boilers, and water heaters...
(AFUE).
Energy Efficiency of heat exchangers
A counter flow heat exchanger is generally, effectively 100% efficient in transferring heat energy from one circuit to the other, albeit at a slight loss in temperature.See also
- Kalina cycleKalina cycleThe Kalina cycle is a thermodynamic process for converting thermal energy into usable mechanical power.It uses a solution of 2 fluids with different boiling points for its working fluid. Since the solution boils over a range of temperatures as in distillation, more of the heat can be extracted...
- Electrical efficiency
- Mechanical efficiencyMechanical efficiencyMechanical efficiency measures the effectiveness of a machine in transforming the energy and power that is input to the device into an output force and movement...
- Heat engineHeat engineIn thermodynamics, a heat engine is a system that performs the conversion of heat or thermal energy to mechanical work. It does this by bringing a working substance from a high temperature state to a lower temperature state. A heat "source" generates thermal energy that brings the working substance...
- Federal Roofing Tax Credit for Energy EfficiencyFederal Roofing Tax Credit for Energy Efficiency-Goals of the Tax Credit:The 2009 roofing tax credit is part of a twofold plan rolled out by the United States Federal government. The first and most relevant of goals was intended to stimulate the economy by incentivizing Americans to replace their roofs with more energy efficient roofs, thereby...
(in the US) - Figure of meritFigure of meritA figure of merit is a quantity used to characterize the performance of a device, system or method, relative to its alternatives. In engineering, figures of merit are often defined for particular materials or devices in order to determine their relative utility for an application...
- Heat of combustionHeat of combustionThe heat of combustion is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon reacting with oxygen to form carbon dioxide, water and heat...
- Lower heating value
- Relative cost of electricity generated by different sources
- Higher heating value
- Energy conversion efficiencyEnergy conversion efficiencyEnergy conversion efficiency is the ratio between the useful output of an energy conversion machine and the input, in energy terms. The useful output may be electric power, mechanical work, or heat.-Overview:...

