In-Methylcyclophane
Encyclopedia
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s and members of a larger family of cyclophane
Cyclophane
A cyclophane is a hydrocarbon consisting of an aromatic unit and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known...
s. These compounds are used to study how chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s in molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s adapt to strain
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
. In-methylcyclophanes in particular have a methyl group in proximity to a benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
ring. This is only possible when both methyl group and ring are attached to the same rigid scaffold. In one In-methylcyclophane molecule this is accomplished with a triptycene
Triptycene
Triptycenes are a class of aromatic hydrocarbons. The parent compound triptycene is the Diels-Alder reaction product of anthracene and benzyne. The compound has a paddlewheel configuration with D3h symmetry...
frame.
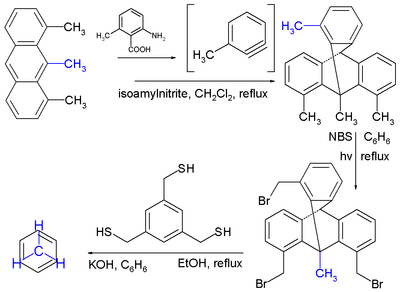
This particular compound is synthesed
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
starting from anthracene
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon consisting of three fused benzene rings. It is a component of coal-tar. Anthracene is used in the production of the red dye alizarin and other dyes...
with a methyl group added to each arene
Arene
Arene or Arênê or Arène may refer to:*an aromatic hydrocarbon*Arene , a genus of marine snails in the family Areneidae*Arene , the wife of Aphareus and mother of Idas and Lynceus in Greek mythology...
ring (1,8,9-trimethylanthracene). A triptycene
Triptycene
Triptycenes are a class of aromatic hydrocarbons. The parent compound triptycene is the Diels-Alder reaction product of anthracene and benzyne. The compound has a paddlewheel configuration with D3h symmetry...
compound is formed from a reaction of this anthracene compound with an aryne
Aryne
In chemistry, an aryne is an uncharged reactive intermediate derived from an aromatic system by removal of two ortho substituents, leaving two orbitals with two electrons distributed between them....
in a Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
in isoamyl nitrite. In this synthesis the precursor to the reactive aryne is 2-amino-6-methylbenzoic acid. Next the methyl substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s are functionalized with bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
groups by the photochemical reaction with N-bromosuccinimide
N-Bromosuccinimide
N-Bromosuccinimide or NBS is a chemical reagent which is used in radical substitution and electrophilic addition reactions in organic chemistry. NBS can be considered a convenient source of cationic bromine.-Preparation:...
or NBS. The final cyclophane is put together by reaction with 1,3,5-tris(mercaptomethyl)benzene with nucleophilic sulfhydryl groups and electrophilic alkyl bromides in a Nucleophilic aliphatic Substitution.
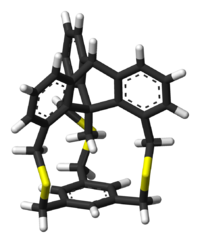
X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
of the tri-sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
derivative of this cyclophane shows that the methyl group located 289.6 picometers from the center of the benzene ring. The carbon to carbon bond linking the methyl group to the triptycene frame is actually shortened and measures 147.5 to 149.5 pm. The similar bond in the triptycene
Triptycene
Triptycenes are a class of aromatic hydrocarbons. The parent compound triptycene is the Diels-Alder reaction product of anthracene and benzyne. The compound has a paddlewheel configuration with D3h symmetry...
precursor is 154 pm. Proton NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
analysis shows a chemical shift
Chemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
of 2.52 ppm for the methyl proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s compared to that of 3.16 to 3.85 in the anthracene compound. The reason for this anomaly is that the methyl protons are in line with the Aromatic ring current
Aromatic ring current
An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring...
of the benzene ring and are therefore severely shielded. In certain paracyclophanes bridging methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
protons can even experience negative chemical shifts in proton NMR.
External links
- Detailed molecular structure Website