Isocitrate dehydrogenase
Encyclopedia
Isocitrate dehydrogenase and , also known as IDH, is an enzyme
that participates in the citric acid cycle
. It catalyzes the third step of the cycle: the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2
while converting NAD+
to NADH
. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol
) to oxalosuccinate (a ketone
), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. Another isoform of the enzyme catalyzes the same reaction, however this reaction is unrelated to the citric acid cycle, is carried out in the cytosol as well as the mitochondrion
and peroxisome
and uses NADP+
as a cofactor instead of NAD+.
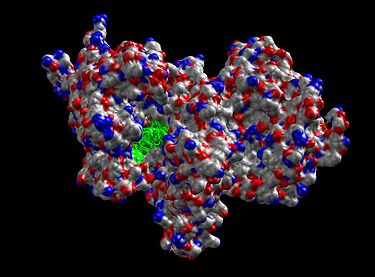 The NAD-IDH is composed of 3 subunits, is allosterically regulated, and requires an integrated Mg2+ or Mn2+ ion. The closest homologue that has a known structure is the E. coli NADP-dependent IDH, which has only 2 subunits and a 13% identity and 29% similarity based on the amino acid sequences, making it dissimilar to human IDH and not suitable for close comparison. All the known NADP-IDHs are homodimers.
The NAD-IDH is composed of 3 subunits, is allosterically regulated, and requires an integrated Mg2+ or Mn2+ ion. The closest homologue that has a known structure is the E. coli NADP-dependent IDH, which has only 2 subunits and a 13% identity and 29% similarity based on the amino acid sequences, making it dissimilar to human IDH and not suitable for close comparison. All the known NADP-IDHs are homodimers.
Most isocitrate dehydrogenases are dimers, to be specific, homodimers (two identical monomer subunits forming one dimeric unit). In comparing C. glutamicum
and E. coli
, monomer and dimer, respectively, both enzymes were found to "efficiently catalyze identical reactions." However, C. glutamicum was recorded as having ten times as much activity than E. coli and seven times more affinitive/specific for NADP. C. glutamicum favored NADP+ over NAD+. In terms of stability with response to temperature, both enzymes had a similar Tm or melting temperature at about 55°C to 60°C. However, the monomer C. glutamicum showed a more consistent stability at higher temperatures, which was expected. The dimer E. coli showed stability at a higher temperature than normal due to the interactions between the two monomeric subunits.
+ or NADP+, Mg2+ / Mn2+ ), product inhibition (by NADH (or NADPH outside the citric acid cycle) and alpha-ketoglutarate), and competitive feedback inhibition (by ATP
).
The overall free energy for this reaction is -8.4 kJ/mol.
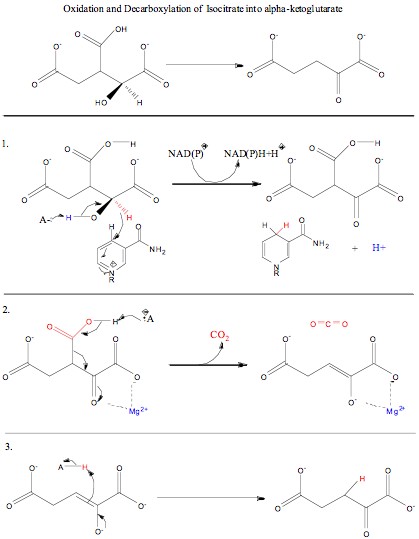
, isocitrate, produced from the isomerization of citrate, undergoes both oxidation and decarboxylation
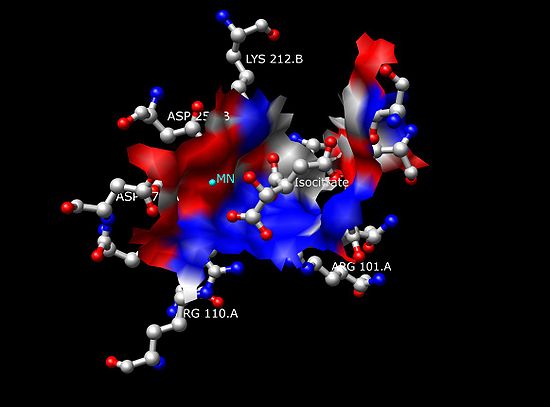
. Using the enzyme Isocitrate Dehydrogenase (IDH), isocitrate is held within its active site
by surrounding arginine
, tyrosine
, asparagine
, serine
, threonine
, and aspartic acid
amino acids. The first box shows the overall isocitrate dehydrogenase reaction. The reactants necessary for this enzyme mechanism to work are isocitrate, NAD+/NADP+, and Mn2+ or Mg2+. The products of the reaction are alpha-ketoglutarate, carbon dioxide
, and NADH + H+/NADPH + H+. Water molecules are used to help deprotonate the oxygens (O3) of isocitrate.
The second box is Step 1, which is the oxidation of the alpha-C (C#2). Oxidation is the first step that isocitrate goes through. In this process, the alcohol
group off the alpha-carbon (C#2) is deprotonated and the electrons flow to the alpha-C forming a ketone
group and removing a hydride
off C#2 using NAD+/NADP+ as an electron accepting cofactor
. The oxidation of the alpha-C allows for a position where electrons (in the next step) will be coming down from the carboxyl group and pushing the electrons (making the double bonded oxygen) back up on the oxygen or grabbing a nearby proton off a nearby Lysine
amino acid.
The third box is Step 2, which is the decarboxylation of oxalosuccinate. In this step, the carboxyl group oxygen is deprotonated by a nearby Tyrosine
amino acid and those electrons flow down to carbon 2. Carbon dioxide leaves the beta carbon of isocitrate as a leaving group with the electrons flowing to the ketone oxygen off the alpha-C placing a negative charge on the oxygen of the alpha-C and forming an alpha-beta unsaturated double bond between carbons 2 and 3. The lone pair
on the alpha-C oxygen picks up a proton
from a nearby Lysine amino acid.
The fourth box is Step 3, which is the saturation of the alpha-beta unsaturated double bond between carbons 2 and 3. In this step of the reaction, Lysine deprotonates the oxygen off the alpha carbon and the lone pair of electrons on the oxygen of the alpha carbon comes down reforming the ketone double bond and pushing the lone pair (forming the double bond between the alpha and beta carbon) off, picking up a proton from the nearby Tyrosine amino acid. This reaction results in the formation of alpha-ketoglutarate, NADH + H+/NADPH + H+, and CO2.
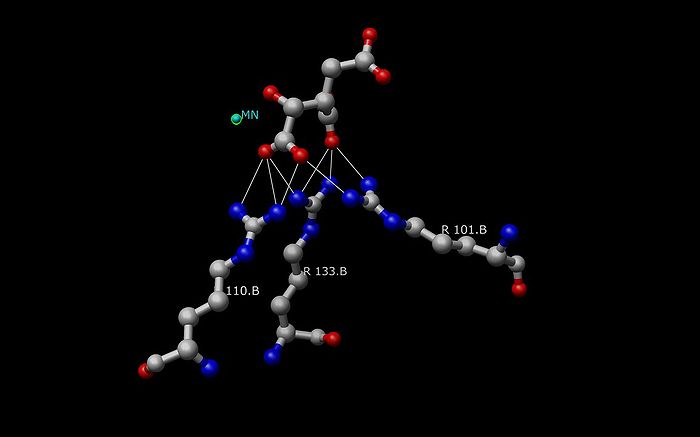
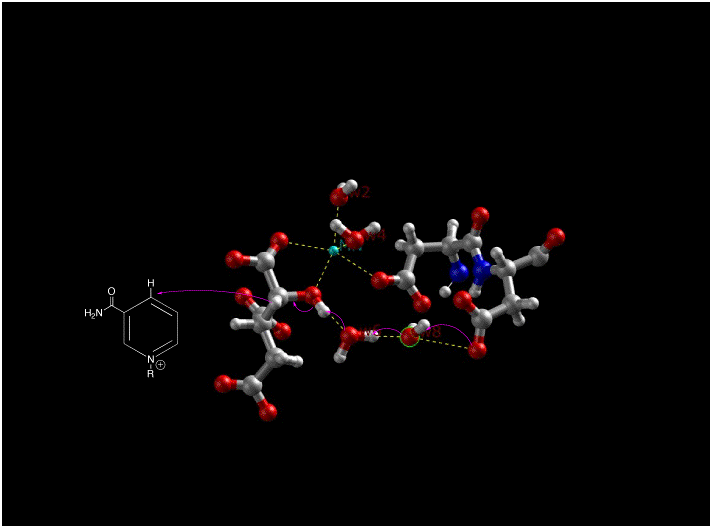
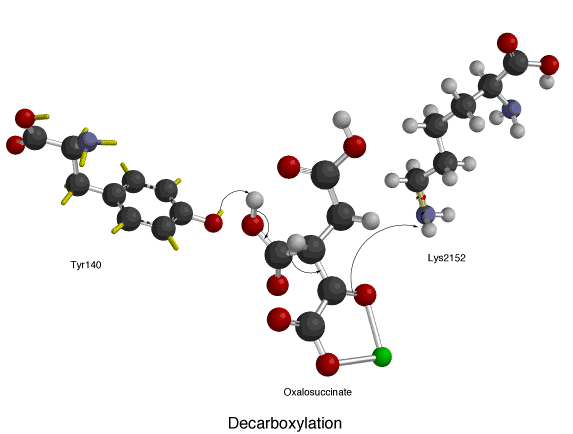
The decarboxylation of oxalosuccinate (below center) is a key step in the formation of alpha-ketoglutarate. In this reaction, the lone pair on the adjacent Tyrosine oxygen pulls off the proton of the carboxyl group. This carboxyl group is also referred to as the beta subunit of isocitrate. The deprotonation of the carboxyl proton causes the lone pair of electrons to move down making carbon dioxide and separating from oxalosuccinate. The electrons continue to move towards the alpha carbon pushing the double bond electrons (making the ketone) up to pull a proton off an adjacent Lysine residue. An alpha-beta unsaturated double bond results between carbon 2 and three. As you can see in the picture, the green ion represents either Mg2+ or Mn2+, which is a cofactor necessary for this reaction to occur. The metal-ion forms a little complex through ionic interactions with the oxygen atoms on the fourth and fifth carbons (also known as the gamma subunit of isocitrate).
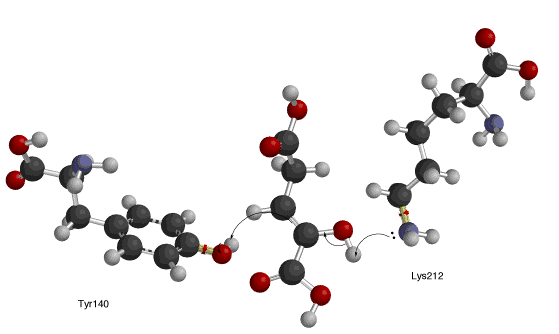
After carbon dioxide is split from the oxalosuccinate in the decarboxylation step (below right), the enol
will retautomerize to the keto from. The reformation of the ketone double bond is started by the deprotonation
of that oxygen off the alpha carbon (C#2) by the same Lysine that protonated the oxygen in the first place. The lone pair of electrons moves down kicking off the lone pairs that were making the double bond. This lone pair of electrons pulls a proton off the Tyrosine that deprotonated the carboxyl group in the decarboxylation step. The reason that we can say that the Lys and Tyr residues will be the same from the previous step because they are helping in holding the isocitrate molecule in the active site of the enzyme. These two residues will be able to hydrogen bond back and forth as long as they’re close enough to the substrate
.
The isocitrate dehydrogenase enzyme as stated above produces alpha-ketoglutarate, carbon dioxide, and NADH + H+/NADPH + H+. There are three changes that occurred throughout the reaction. The oxidation of Carbon 2, the decarboxylation (loss of carbon dioxide) off Carbon 3, and the formation of a ketone group with a stereochemical change from sp3 to sp2.
 The Isocitrate Dehydrogenase (IDH) enzyme structure in Escherichia coli was the first structure to be built and understood. Since then, Escherichia coli has been used by most researchers to make comparisons to other isocitrate dehydrogenase enzymes. There is much detailed knowledge about this bacterial enzyme, and it has been found that most isocitrate dehydrogenases are similar in structure and therefore function. This similarity of structure and function gives reason to believe that the structures are conserved as well as the amino acids. Therefore, the active sites amongst most prokaryotic isocitrate dehydrogenase enzymes should be conserved as well, which is observed throughout many studies done on prokaryotic enzymes. Eukaryotic isocitrate dehydrogenase enzymes on the other hand, have not been fully discovered yet.
The Isocitrate Dehydrogenase (IDH) enzyme structure in Escherichia coli was the first structure to be built and understood. Since then, Escherichia coli has been used by most researchers to make comparisons to other isocitrate dehydrogenase enzymes. There is much detailed knowledge about this bacterial enzyme, and it has been found that most isocitrate dehydrogenases are similar in structure and therefore function. This similarity of structure and function gives reason to believe that the structures are conserved as well as the amino acids. Therefore, the active sites amongst most prokaryotic isocitrate dehydrogenase enzymes should be conserved as well, which is observed throughout many studies done on prokaryotic enzymes. Eukaryotic isocitrate dehydrogenase enzymes on the other hand, have not been fully discovered yet.
Each dimer of IDH has two active sites. Each active site binds a NAD+/NADP+ molecule and a metal ion (Mg2+,Mn2+). In general, each active site has a conserved sequence of amino acids for each specific binding site. In Desulfotalea psychrophila (DpIDH) and porcine (PcIDH) there are three substrates bound to the active site.
, oligodendroglioma
and glioblastoma multiforme
, with mutations found in nearly all cases of secondary glioblastomas, which develop from lower-grade gliomas, but rarely in primary high-grade glioblastoma multiforme
. Patients whose tumor had an IDH1 mutation had longer survival. It is not known how the IDH1 mutation contributes to development of glioblastoma multiforme
. Furthermore mutations of IDH2 and IDH1 were found in up to 20% of cytogenetically normal acute myeloid leukemia
(AML). These mutations are known to produce (D)-2-hydroxyglutarate from alpha-ketoglutarate. Somatic mosaic mutations of this gene have also been found associated to Ollier disease and Maffucci syndrome .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that participates in the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
. It catalyzes the third step of the cycle: the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
while converting NAD+
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
to NADH
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
) to oxalosuccinate (a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. Another isoform of the enzyme catalyzes the same reaction, however this reaction is unrelated to the citric acid cycle, is carried out in the cytosol as well as the mitochondrion
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
and peroxisome
Peroxisome
Peroxisomes are organelles found in virtually all eukaryotic cells. They are involved in the catabolism of very long chain fatty acids, branched chain fatty acids, D-amino acids, polyamines, and biosynthesis of plasmalogens, etherphospholipids critical for the normal function of mammalian brains...
and uses NADP+
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
as a cofactor instead of NAD+.
NADP+ dependent
Each NADP+-dependent isozyme functions as a homodimer:NAD+ dependent
The isocitrate dehydrogenase 3 isozyme is a heterotetramer that is composed of two alpha subunits, one beta subunit, and one gamma subunit:Structure

Most isocitrate dehydrogenases are dimers, to be specific, homodimers (two identical monomer subunits forming one dimeric unit). In comparing C. glutamicum
Corynebacterium
Corynebacterium is a genus of Gram-positive rod-shaped bacteria. They are widely distributed in nature and are mostly innocuous. Some are useful in industrial settings such as C. glutamicum. Others can cause human disease. C...
and E. coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
, monomer and dimer, respectively, both enzymes were found to "efficiently catalyze identical reactions." However, C. glutamicum was recorded as having ten times as much activity than E. coli and seven times more affinitive/specific for NADP. C. glutamicum favored NADP+ over NAD+. In terms of stability with response to temperature, both enzymes had a similar Tm or melting temperature at about 55°C to 60°C. However, the monomer C. glutamicum showed a more consistent stability at higher temperatures, which was expected. The dimer E. coli showed stability at a higher temperature than normal due to the interactions between the two monomeric subunits.
Regulation
The IDH step of the citric acid cycle, due to its large negative free energy change, is one of the irreversible reactions in the citric acid cycle, and, therefore, must be carefully regulated to avoid unnecessary depletion of isocitrate (and therefore an accumulation of alpha-ketoglutarate). The reaction is stimulated by the simple mechanisms of substrate availability (isocitrate, NADNicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
+ or NADP+, Mg2+ / Mn2+ ), product inhibition (by NADH (or NADPH outside the citric acid cycle) and alpha-ketoglutarate), and competitive feedback inhibition (by ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
).
Catalytic mechanism
Isocitrate + NADP+ Mg2+(metal ion) --> alpha-ketoglutarate + NADPH + H+ + CO2The overall free energy for this reaction is -8.4 kJ/mol.

Steps
Within the citric acid cycleCitric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
, isocitrate, produced from the isomerization of citrate, undergoes both oxidation and decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
. Using the enzyme Isocitrate Dehydrogenase (IDH), isocitrate is held within its active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
by surrounding arginine
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
, tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, asparagine
Asparagine
Asparagine is one of the 20 most common natural amino acids on Earth. It has carboxamide as the side-chain's functional group. It is not an essential amino acid...
, serine
Serine
Serine is an amino acid with the formula HO2CCHCH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.-Occurrence and biosynthesis:...
, threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
, and aspartic acid
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
amino acids. The first box shows the overall isocitrate dehydrogenase reaction. The reactants necessary for this enzyme mechanism to work are isocitrate, NAD+/NADP+, and Mn2+ or Mg2+. The products of the reaction are alpha-ketoglutarate, carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, and NADH + H+/NADPH + H+. Water molecules are used to help deprotonate the oxygens (O3) of isocitrate.
The second box is Step 1, which is the oxidation of the alpha-C (C#2). Oxidation is the first step that isocitrate goes through. In this process, the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
group off the alpha-carbon (C#2) is deprotonated and the electrons flow to the alpha-C forming a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
group and removing a hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
off C#2 using NAD+/NADP+ as an electron accepting cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
. The oxidation of the alpha-C allows for a position where electrons (in the next step) will be coming down from the carboxyl group and pushing the electrons (making the double bonded oxygen) back up on the oxygen or grabbing a nearby proton off a nearby Lysine
Lysine
Lysine is an α-amino acid with the chemical formula HO2CCH4NH2. It is an essential amino acid, which means that the human body cannot synthesize it. Its codons are AAA and AAG....
amino acid.
The third box is Step 2, which is the decarboxylation of oxalosuccinate. In this step, the carboxyl group oxygen is deprotonated by a nearby Tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
amino acid and those electrons flow down to carbon 2. Carbon dioxide leaves the beta carbon of isocitrate as a leaving group with the electrons flowing to the ketone oxygen off the alpha-C placing a negative charge on the oxygen of the alpha-C and forming an alpha-beta unsaturated double bond between carbons 2 and 3. The lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
on the alpha-C oxygen picks up a proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
from a nearby Lysine amino acid.
The fourth box is Step 3, which is the saturation of the alpha-beta unsaturated double bond between carbons 2 and 3. In this step of the reaction, Lysine deprotonates the oxygen off the alpha carbon and the lone pair of electrons on the oxygen of the alpha carbon comes down reforming the ketone double bond and pushing the lone pair (forming the double bond between the alpha and beta carbon) off, picking up a proton from the nearby Tyrosine amino acid. This reaction results in the formation of alpha-ketoglutarate, NADH + H+/NADPH + H+, and CO2.
Detailed mechanism
Two aspartate amino acid residues (below left) are interacting with two adjacent water molecules (w6 and w8) in the Mn2+ isocitrate porcine IDH complex to deprotonate the alcohol off the alpha-carbon atom. The oxidation of the alpha-C also takes place in this picture where NAD+ accepts a hydride resulting in oxalosuccinate. Along with the sp3 to sp2 stereochemical change around the alpha-C, there is a ketone group that is formed form the alcohol group. The formation of this ketone double bond allows for resonance to take place as electrons coming down from the leaving carboxylate group move towards the ketone.The decarboxylation of oxalosuccinate (below center) is a key step in the formation of alpha-ketoglutarate. In this reaction, the lone pair on the adjacent Tyrosine oxygen pulls off the proton of the carboxyl group. This carboxyl group is also referred to as the beta subunit of isocitrate. The deprotonation of the carboxyl proton causes the lone pair of electrons to move down making carbon dioxide and separating from oxalosuccinate. The electrons continue to move towards the alpha carbon pushing the double bond electrons (making the ketone) up to pull a proton off an adjacent Lysine residue. An alpha-beta unsaturated double bond results between carbon 2 and three. As you can see in the picture, the green ion represents either Mg2+ or Mn2+, which is a cofactor necessary for this reaction to occur. The metal-ion forms a little complex through ionic interactions with the oxygen atoms on the fourth and fifth carbons (also known as the gamma subunit of isocitrate).
After carbon dioxide is split from the oxalosuccinate in the decarboxylation step (below right), the enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
will retautomerize to the keto from. The reformation of the ketone double bond is started by the deprotonation
Deprotonation
Deprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of that oxygen off the alpha carbon (C#2) by the same Lysine that protonated the oxygen in the first place. The lone pair of electrons moves down kicking off the lone pairs that were making the double bond. This lone pair of electrons pulls a proton off the Tyrosine that deprotonated the carboxyl group in the decarboxylation step. The reason that we can say that the Lys and Tyr residues will be the same from the previous step because they are helping in holding the isocitrate molecule in the active site of the enzyme. These two residues will be able to hydrogen bond back and forth as long as they’re close enough to the substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
.
 |
 |
 |
The isocitrate dehydrogenase enzyme as stated above produces alpha-ketoglutarate, carbon dioxide, and NADH + H+/NADPH + H+. There are three changes that occurred throughout the reaction. The oxidation of Carbon 2, the decarboxylation (loss of carbon dioxide) off Carbon 3, and the formation of a ketone group with a stereochemical change from sp3 to sp2.
 |
 |
 |
 |
Active site

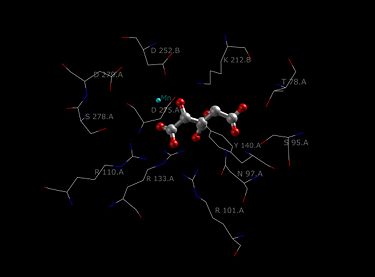
Each dimer of IDH has two active sites. Each active site binds a NAD+/NADP+ molecule and a metal ion (Mg2+,Mn2+). In general, each active site has a conserved sequence of amino acids for each specific binding site. In Desulfotalea psychrophila (DpIDH) and porcine (PcIDH) there are three substrates bound to the active site.
- Isocitrate binds within the active site to a conserved sequence of about eight amino acids through hydrogen bonds. These acids include (may vary in residue but with similar properties) tyrosine, serine, asparagine, arginine, arginine, arginine, tyrosine, and lysine. Their positions on the backbone vary but they are all within a close range (i.e. Arg131 DpIDH and Arg133 PcIDH, Tyr138 DpIDH and Tyr140 PcIDH).
- The metal ion (Mg2+, Mn2+) binds to three conserved amino acids through hydrogen bonds. These amino acids include three Aspartate residues.
- NAD+ and NADP+ bind within the active site within four regions with similar properties amongst IDH enzymes. These regions vary but are around [250-260], [280-290], [300-330], and [365-380]. Again regions vary but the proximity of regions are conserved.
Clinical significance
Specific mutations in the isocitrate dehydrogenase gene IDH1 have been found in several brain tumors including astrocytomaAstrocytoma
Astrocytomas are a type of neoplasm of the brain. They originate in a particular kind of glial-cells, star-shaped brain cells in the cerebrum called astrocytes. This type of tumor does not usually spread outside the brain and spinal cord and it does not usually affect other organs...
, oligodendroglioma
Oligodendroglioma
Oligodendrogliomas are a type of glioma that are believed to originate from the oligodendrocytes of the brain or from a glial precursor cell. They occur primarily in adults but are also found in children...
and glioblastoma multiforme
Glioblastoma multiforme
Glioblastoma multiforme is the most common and most aggressive malignant primary brain tumor in humans, involving glial cells and accounting for 52% of all functional tissue brain tumor cases and 20% of all intracranial tumors. Despite being the most prevalent form of primary brain tumor, GBMs...
, with mutations found in nearly all cases of secondary glioblastomas, which develop from lower-grade gliomas, but rarely in primary high-grade glioblastoma multiforme
Glioblastoma multiforme
Glioblastoma multiforme is the most common and most aggressive malignant primary brain tumor in humans, involving glial cells and accounting for 52% of all functional tissue brain tumor cases and 20% of all intracranial tumors. Despite being the most prevalent form of primary brain tumor, GBMs...
. Patients whose tumor had an IDH1 mutation had longer survival. It is not known how the IDH1 mutation contributes to development of glioblastoma multiforme
Glioblastoma multiforme
Glioblastoma multiforme is the most common and most aggressive malignant primary brain tumor in humans, involving glial cells and accounting for 52% of all functional tissue brain tumor cases and 20% of all intracranial tumors. Despite being the most prevalent form of primary brain tumor, GBMs...
. Furthermore mutations of IDH2 and IDH1 were found in up to 20% of cytogenetically normal acute myeloid leukemia
Acute myeloid leukemia
Acute myeloid leukemia , also known as acute myelogenous leukemia, is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal white blood cells that accumulate in the bone marrow and interfere with the production of normal blood cells. AML is the most common acute...
(AML). These mutations are known to produce (D)-2-hydroxyglutarate from alpha-ketoglutarate. Somatic mosaic mutations of this gene have also been found associated to Ollier disease and Maffucci syndrome .

