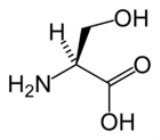
Serine
Encyclopedia
Serine is an amino acid
with the formula
H
O
2C
CH(N
H2)CH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.
s. Its codons are UCU, UCC, UCA, UCG, AGU and AGC. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine
. Serine was first obtained from silk
protein, a particularly rich source, in 1865. Its name is derived from the Latin
for silk, sericum. Serine's structure was established in 1902.
The biosynthesis of serine starts with the oxidation of 3-phosphoglycerate to 3-phosphohydroxypyruvate and NADH. Reductive amination
of this ketone followed by hydrolysis gives serine. Serine hydroxymethyltransferase
catalyzes the reversible, simultaneous conversions of L-serine to glycine
(retro-aldol cleavage) and 5,6,7,8-tetrahydrofolate to 5,10-methylenetetrahydrofolate
(hydrolysis).
This compound may also be naturally produced when UV light illuminates simple ices such as a combination of water, methanol, hydrogen cyanide, and ammonia, suggesting that it may be easily produced in cold regions of space.
via several steps:
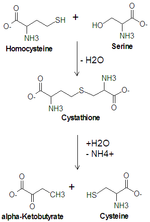 Serine is important in metabolism
Serine is important in metabolism
in that it participates in the biosynthesis
of purines and pyrimidines. It is the precursor to several amino acids including glycine
and cysteine
, and tryptophan
in bacteria. It is also the precursor to numerous other metabolites, including sphingolipid
s and folate, which is the principal donor of one-carbon fragments in biosynthesis.
s. It has been shown to occur in the active sites of chymotrypsin
, trypsin
, and many other enzymes. The so-called nerve gases and many substances used in insecticide
s have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely.
As a constituent (residue) of proteins, its side chain
can undergo O-linked glycosylation
, which may be functionally related to diabetes.
It is one of three amino acid residues that are commonly phosphorylated
by kinases during cell signaling
in eukaryote
s. Phosphorylated serine residues are often referred to as phosphoserine
.
Serine protease
s are a common type of protease.
from L-serine (its enantiomer
), serves as both a neurotransmitter
and a gliotransmitter
by activating NMDA receptor
s, making them able to open if they then also bind glutamate. D-serine is a potent agonist
at the glycine
site of the NMDA
-type glutamate receptor
. For the receptor to open, glutamate and either glycine or D-serine must bind to it. In fact, D-serine is a more potent agonist at the glycine site on the NMDAR than glycine itself. D-serine was only thought to exist in bacteria until relatively recently; it was the second D amino acid discovered to naturally exist in humans, present as a signalling molecule in the brain, soon after the discovery of D-aspartate. Had D amino acids been discovered in humans sooner, the glycine site on the NMDA receptor might instead be named the D-serine site.
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
2C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
CH(N
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
H2)CH2OH. It is one of the proteinogenic amino acids. By virtue of the hydroxyl group, serine is classified as a polar amino acid.
Occurrence and biosynthesis
This compound is one of the naturally occurring proteinogenic amino acidProteinogenic amino acid
Proteinogenic amino acids are those amino acids that can be found in proteins and require cellular machinery coded for in the genetic code of any organism for their isolated production. There are 22 standard amino acids, but only 21 are found in eukaryotes. Of the 22, 20 are directly encoded by...
s. Its codons are UCU, UCC, UCA, UCG, AGU and AGC. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
. Serine was first obtained from silk
Silk
Silk is a natural protein fiber, some forms of which can be woven into textiles. The best-known type of silk is obtained from the cocoons of the larvae of the mulberry silkworm Bombyx mori reared in captivity...
protein, a particularly rich source, in 1865. Its name is derived from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
for silk, sericum. Serine's structure was established in 1902.
The biosynthesis of serine starts with the oxidation of 3-phosphoglycerate to 3-phosphohydroxypyruvate and NADH. Reductive amination
Reductive amination
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine...
of this ketone followed by hydrolysis gives serine. Serine hydroxymethyltransferase
Serine hydroxymethyltransferase
Serine hydroxymethyltransferase is an enzyme which plays an important role in cellular one-carbon pathways by catalyzing the reversible, simultaneous conversions of L-serine to glycine and tetrahydrofolate to 5,10-methylenetetrahydrofolate...
catalyzes the reversible, simultaneous conversions of L-serine to glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
(retro-aldol cleavage) and 5,6,7,8-tetrahydrofolate to 5,10-methylenetetrahydrofolate
5,10-Methylenetetrahydrofolate
5,10-Methylenetetrahydrofolate is the substrate used by the enzyme methylenetetrahydrofolate reductase to generate 5-methyltetrahydrofolate ....
(hydrolysis).
This compound may also be naturally produced when UV light illuminates simple ices such as a combination of water, methanol, hydrogen cyanide, and ammonia, suggesting that it may be easily produced in cold regions of space.
Production
Industrially, L-serine is produced by fermentation, with an estimated 100-1000 tonnes per year produced. In the laboratory, racemic serine can be prepared from methyl acrylateMethyl acrylate
Methyl acrylate is a volatile chemical compound classified as a methyl ester. It has a characteristic acrid odor used in the preparation of polyamidoamine dendrimers typically by Michael addition with a primary amine....
via several steps:
Metabolic

Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
in that it participates in the biosynthesis
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
of purines and pyrimidines. It is the precursor to several amino acids including glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
and cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
, and tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
in bacteria. It is also the precursor to numerous other metabolites, including sphingolipid
Sphingolipid
Sphingolipids are a class of lipids containing a backbone of sphingoid bases, a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named for the mythological Sphinx because of their enigmatic nature. These compounds play...
s and folate, which is the principal donor of one-carbon fragments in biosynthesis.
Structural role
Serine plays an important role in the catalytic function of many enzymeEnzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s. It has been shown to occur in the active sites of chymotrypsin
Chymotrypsin
Chymotrypsin is a digestive enzyme that can perform proteolysis. Chymotrypsin preferentially cleaves peptide amide bonds where the carboxyl side of the amide bond is a tyrosine, tryptophan, or phenylalanine. These amino acids contain an aromatic ring in their sidechain that fits into a...
, trypsin
Trypsin
Trypsin is a serine protease found in the digestive system of many vertebrates, where it hydrolyses proteins. Trypsin is produced in the pancreas as the inactive proenzyme trypsinogen. Trypsin cleaves peptide chains mainly at the carboxyl side of the amino acids lysine or arginine, except when...
, and many other enzymes. The so-called nerve gases and many substances used in insecticide
Insecticide
An insecticide is a pesticide used against insects. They include ovicides and larvicides used against the eggs and larvae of insects respectively. Insecticides are used in agriculture, medicine, industry and the household. The use of insecticides is believed to be one of the major factors behind...
s have been shown to act by combining with a residue of serine in the active site of acetylcholine esterase, inhibiting the enzyme completely.
As a constituent (residue) of proteins, its side chain
Side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called "main chain" or backbone. The placeholder R is often used as a generic placeholder for alkyl group side chains in chemical structure diagrams. To indicate other non-carbon...
can undergo O-linked glycosylation
Glycosylation
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule . In biology glycosylation refers to the enzymatic process that attaches glycans to proteins, lipids, or other organic molecules...
, which may be functionally related to diabetes.
It is one of three amino acid residues that are commonly phosphorylated
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
by kinases during cell signaling
Signal transduction
Signal transduction occurs when an extracellular signaling molecule activates a cell surface receptor. In turn, this receptor alters intracellular molecules creating a response...
in eukaryote
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
s. Phosphorylated serine residues are often referred to as phosphoserine
Phosphoserine
Phosphoserine is an ester of serine and phosphoric acid. Phosphoserine is a component of many proteins as the result of posttranslational modifications. The phosphorylation of the alcohol functional group in serine to produce phosphoserine is catalyzed by various types of kinases....
.
Serine protease
Serine protease
Serine proteases are enzymes that cleave peptide bonds in proteins, in which serine serves as the nucleophilic amino acid at the active site.They are found ubiquitously in both eukaryotes and prokaryotes...
s are a common type of protease.
Signaling
D-Serine, synthesized in the brain by serine racemaseSerine racemase
Serine racemase is an enzyme which generates D-serine from L-serine. D-serine acts as a neuronal signaling molecule by activating NMDA receptors in the brain...
from L-serine (its enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
), serves as both a neurotransmitter
Neurotransmitter
Neurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
and a gliotransmitter
Gliotransmitter
Gliotransmitters are chemicals released from glial cells that facilitate neuronal communication between neurons and other glial cells and are usually induced from Ca2+ signaling. [3] While gliotransmitters can be released from any glial cell, including oligodendrocytes, astrocytes, and microglia,...
by activating NMDA receptor
NMDA receptor
The NMDA receptor , a glutamate receptor, is the predominant molecular device for controlling synaptic plasticity and memory function....
s, making them able to open if they then also bind glutamate. D-serine is a potent agonist
Agonist
An agonist is a chemical that binds to a receptor of a cell and triggers a response by that cell. Agonists often mimic the action of a naturally occurring substance...
at the glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
site of the NMDA
NMDA
N-Methyl-D-aspartic acid or N-Methyl-D-aspartate is an amino acid derivative which acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor...
-type glutamate receptor
Glutamate receptor
Glutamate receptors are synaptic receptors located primarily on the membranes of neuronal cells. Glutamate is one of the 20 amino acids used to assemble proteins and as a result is abundant in many areas of the body, but it also functions as a neurotransmitter and is particularly abundant in the...
. For the receptor to open, glutamate and either glycine or D-serine must bind to it. In fact, D-serine is a more potent agonist at the glycine site on the NMDAR than glycine itself. D-serine was only thought to exist in bacteria until relatively recently; it was the second D amino acid discovered to naturally exist in humans, present as a signalling molecule in the brain, soon after the discovery of D-aspartate. Had D amino acids been discovered in humans sooner, the glycine site on the NMDA receptor might instead be named the D-serine site.

