
Isotopes of americium
Encyclopedia
Americium
(Am) is an artificial element, and thus a standard atomic mass
cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope
to be synthesized was 241Am in 1944.
19 radioisotopes of americium have been characterized, with the most stable being 243Am with a half-life
of 7,370 years, and 241Am with a half-life of 432.7 years. All of the remaining radioactive
isotopes have half-lives that are less than 51 hours, and the majority of these have half-lives that are less than 100 minutes. This element also has 8 meta states, with the most stable being 242mAm (t½ 141 years). The isotopes of americium range in atomic weight
from 231.046 u
(231Am) to 249.078 u (249Am).
based an ionization chamber
. It is a potential fuel for long-lifetime radioisotope thermoelectric generator
s.
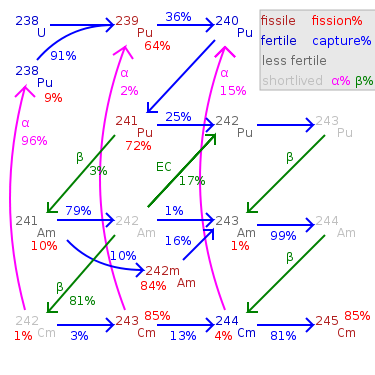
Possible parent nuclides: beta from 241Pu
, electron capture from 241Cm, alpha from 245Bk.
Americium-241 decays by alpha emission, with a by-product of gamma ray
s. Its presence in plutonium
is determined by the original concentration of plutonium-241 and the sample age. Because of the low penetration of alpha radiation, Americium-241 only poses a health risk when ingested or inhaled. Older samples of plutonium containing plutonium-241 contain a buildup of 241Am. A chemical removal of americium from reworked plutonium, e.g. during reworking of plutonium pits, may be required.
Americium-242m has a mass of 242.0595492 g/mol. It is one of the rare cases, like 180mTa, where a higher-energy nuclear isomer
is more stable than the lower-energy one, Americium-242.
242mAm is fissile
(because it has an odd number of neutron
s) and has a low critical mass
, comparable to that of 239Pu
. It has a very high cross section
for fission, and if in a nuclear reactor is destroyed relatively quickly.
Another report claims that 242mAm has a much lower critical mass, can sustain a chain reaction even as a thin film, and could be used for a novel type of nuclear rocket.
of 7,370 years, the longest lasting of all americium isotopes. It is formed in the nuclear fuel cycle
by neutron capture
on plutonium-242
followed by beta decay
. Production increases exponentially with increasing burnup
as a total of 5 neutron captures on 238U
are required.
It decays by either emitting an alpha particle
(with a decay energy of 5.27MeV) to become 239Np, which then quickly decays to 239Pu
, or infrequently, by spontaneous fission
.
243Am is a hazardous substance, because it can cause cancer. 239Np, which is formed from 243Am, emits dangerous gamma rays, making 243Am the most dangerous isotope of Americium.
Americium
Americium is a synthetic element that has the symbol Am and atomic number 95. This transuranic element of the actinide series is located in the periodic table below the lanthanide element europium, and thus by analogy was named after another continent, America.Americium was first produced in 1944...
(Am) is an artificial element, and thus a standard atomic mass
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
to be synthesized was 241Am in 1944.
19 radioisotopes of americium have been characterized, with the most stable being 243Am with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 7,370 years, and 241Am with a half-life of 432.7 years. All of the remaining radioactive
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
isotopes have half-lives that are less than 51 hours, and the majority of these have half-lives that are less than 100 minutes. This element also has 8 meta states, with the most stable being 242mAm (t½ 141 years). The isotopes of americium range in atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
from 231.046 u
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
(231Am) to 249.078 u (249Am).
Americium-241
Americium-241 is the most prevalent isotope of americium in nuclear waste. It is the americium isotope used in an americium smoke detectorAmericium smoke detector
An americium smoke detector is a smoke detector that uses americium-241 to detect the changes in the flow of ions that indicates the presence of smoke...
based an ionization chamber
Ionization chamber
The ionization chamber is the simplest of all gas-filled radiation detectors, and is used for the detection or measurement of ionizing radiation...
. It is a potential fuel for long-lifetime radioisotope thermoelectric generator
Radioisotope thermoelectric generator
A radioisotope thermoelectric generator is an electrical generator that obtains its power from radioactive decay. In such a device, the heat released by the decay of a suitable radioactive material is converted into electricity by the Seebeck effect using an array of thermocouples.RTGs can be...
s.
| Parameter | Value |
|---|---|
| Atomic mass Atomic mass The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom.... |
241.056823 u Atomic mass unit The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of... |
| Mass excess Mass excess The mass excess of a nuclide is the difference between its actual mass and its mass number in atomic mass units. It is one of the predominant methods for tabulating nuclear mass. The mass of an atomic nucleus is well approximated by its mass number, which indicates that most of the mass of a... |
52930 keV Electronvolt In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt... |
| Beta decay Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... energy Decay energy The decay energy is the energy released by a radioactive decay. Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting ionizing particles and radiation... |
-767 keV |
| Spin Spin (physics) In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,... |
5/2- |
| Half-life Half-life Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to... |
432.2 years |
| Spontaneous fission Spontaneous fission Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses... s |
1200 per kg/s |
| Decay heat Decay heat Decay heat is the heat released as a result of radioactive decay. This is when the radiation interacts with materials: the energy of the alpha, beta or gamma radiation is converted into the thermal movement of atoms.-Natural occurrence:... |
114 watts/kg |
Possible parent nuclides: beta from 241Pu
Plutonium-241
Plutonium-241 is an isotope of plutonium formed when plutonium-240 captures a neutron. Like Pu-239 but unlike 240Pu, 241Pu is fissile, with a neutron absorption cross section about 1/3 greater than 239Pu, and a similar probability of fissioning on neutron absorption, around 73%. In the non-fission...
, electron capture from 241Cm, alpha from 245Bk.
Americium-241 decays by alpha emission, with a by-product of gamma ray
Gamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
s. Its presence in plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
is determined by the original concentration of plutonium-241 and the sample age. Because of the low penetration of alpha radiation, Americium-241 only poses a health risk when ingested or inhaled. Older samples of plutonium containing plutonium-241 contain a buildup of 241Am. A chemical removal of americium from reworked plutonium, e.g. during reworking of plutonium pits, may be required.
Americium-242m

| Probability | Decay mode | Decay energy Decay energy The decay energy is the energy released by a radioactive decay. Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting ionizing particles and radiation... | Decay product Decay product In nuclear physics, a decay product is the remaining nuclide left over from radioactive decay. Radioactive decay often involves a sequence of steps... |
|---|---|---|---|
| 99.54% | isomeric transition Isomeric transition An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer.... |
0.05 MeV MEV MeV and meV are multiples and submultiples of the electron volt unit referring to 1,000,000 eV and 0.001 eV, respectively.Mev or MEV may refer to:In entertainment:* Musica Elettronica Viva, an Italian musical group... |
242Am |
| 0.46% | alpha decay Alpha decay Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less... |
5.64 MeV | 238Np |
| (1.5±0.6)×10−10 | spontaneous fission Spontaneous fission Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses... |
~200 MeV | fission product Fission product Nuclear fission products are the atomic fragments left after a large atomic nucleus fissions. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons and a large release of energy in the form of heat , gamma rays and neutrinos. The... s |
Americium-242m has a mass of 242.0595492 g/mol. It is one of the rare cases, like 180mTa, where a higher-energy nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
is more stable than the lower-energy one, Americium-242.
242mAm is fissile
Fissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
(because it has an odd number of neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s) and has a low critical mass
Critical mass
A critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties A critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The...
, comparable to that of 239Pu
Plutonium-239
Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in...
. It has a very high cross section
Nuclear cross section
The nuclear cross section of a nucleus is used to characterize the probability that a nuclear reaction will occur. The concept of a nuclear cross section can be quantified physically in terms of "characteristic area" where a larger area means a larger probability of interaction...
for fission, and if in a nuclear reactor is destroyed relatively quickly.
Another report claims that 242mAm has a much lower critical mass, can sustain a chain reaction even as a thin film, and could be used for a novel type of nuclear rocket.
| Probability | Decay mode | Decay energy Decay energy The decay energy is the energy released by a radioactive decay. Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting ionizing particles and radiation... | Decay product Decay product In nuclear physics, a decay product is the remaining nuclide left over from radioactive decay. Radioactive decay often involves a sequence of steps... |
|---|---|---|---|
| 82.70% | beta decay Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... |
0.665 MeV | 242Cm Curium Curium is a synthetic chemical element with the symbol Cm and atomic number 96. This radioactive transuranic element of the actinide series was named after Marie Skłodowska-Curie and her husband Pierre Curie. Curium was first intentionally produced and identified in summer 1944 by the group of... |
| 17.30% | electron capture Electron capture Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino... |
0.751 MeV | 242Pu Plutonium-242 Pu-242 is one of the isotopes of plutonium, the second longest-lived, with a half-life of 373,300 years.242Pu's halflife is about 15 times as long as Pu-239's halflife; therefore it is 1/15 as radioactive and not one of the larger contributors to nuclear waste radioactivity.242Pu's gamma ray... |
Americium-243
Americium-243 has a mass of 243.06138 g/mol and a half-lifeHalf-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 7,370 years, the longest lasting of all americium isotopes. It is formed in the nuclear fuel cycle
Nuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in...
by neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
on plutonium-242
Plutonium-242
Pu-242 is one of the isotopes of plutonium, the second longest-lived, with a half-life of 373,300 years.242Pu's halflife is about 15 times as long as Pu-239's halflife; therefore it is 1/15 as radioactive and not one of the larger contributors to nuclear waste radioactivity.242Pu's gamma ray...
followed by beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
. Production increases exponentially with increasing burnup
Burnup
In nuclear power technology, burnup is a measure of how much energy is extracted from a primary nuclear fuel source...
as a total of 5 neutron captures on 238U
Uranium-238
Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239...
are required.
It decays by either emitting an alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
(with a decay energy of 5.27MeV) to become 239Np, which then quickly decays to 239Pu
Plutonium-239
Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in...
, or infrequently, by spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
.
243Am is a hazardous substance, because it can cause cancer. 239Np, which is formed from 243Am, emits dangerous gamma rays, making 243Am the most dangerous isotope of Americium.
Table
| nuclide symbol |
Z(p Proton The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number.... ) |
N(n Neutron The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of... ) |
isotopic mass (u) |
half-life | decay mode(s)Abbreviations: CD: Cluster decay Cluster decay Cluster decay is a type of nuclear decay in which a parent atomic nucleus with A nucleons and Z protons emits a cluster of Ne neutrons and Ze protons heavier than an alpha particle but lighter than a typical binary fission fragment Cluster decay (also named heavy particle radioactivity or heavy... EC: Electron capture Electron capture Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino... IT: Isomeric transition Isomeric transition An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer.... SF: Spontaneous fission Spontaneous fission Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses... |
daughter isotope(s) |
nuclear spin |
|---|---|---|---|---|---|---|---|
| excitation energy | |||||||
| 231Am | 95 | 136 | 231.04556(32)# | 30# s | β+ Beta decay In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a... |
231Pu | |
| α Alpha decay Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less... (rare) |
227Np | ||||||
| 232Am | 95 | 137 | 232.04659(32)# | 79(2) s | β+ (98%) | 232Pu | |
| α (2%) | 228Np | ||||||
| β+, SF Spontaneous fission Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses... (.069%) |
(various) | ||||||
| 233Am | 95 | 138 | 233.04635(11)# | 3.2(8) min | β+ | 233Pu | |
| α | 229Np | ||||||
| 234Am | 95 | 139 | 234.04781(22)# | 2.32(8) min | β+ (99.95%) | 234Pu | |
| α (.04%) | 230Np | ||||||
| β+, SF (.0066%) | (various) | ||||||
| 235Am | 95 | 140 | 235.04795(13)# | 9.9(5) min | β+ | 235Pu | 5/2-# |
| α (rare) | 231Np | ||||||
| 236Am | 95 | 141 | 236.04958(11)# | 3.6(1) min | β+ | 236Pu | |
| α | 232Np | ||||||
| 237Am | 95 | 142 | 237.05000(6)# | 73.0(10) min | β+ (99.97%) | 237Pu | 5/2(-) |
| α (.025%) | 233Np | ||||||
| 238Am | 95 | 143 | 238.05198(5) | 98(2) min | β+ | 238Pu | 1+ |
| α (10−4%) | 234Np | ||||||
| 238mAm | 2500(200)# keV | 35(10) µs | |||||
| 239Am | 95 | 144 | 239.0530245(26) | 11.9(1) h | EC Electron capture Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino... (99.99%) |
236Pu | (5/2)- |
| α (.01%) | 235Np | ||||||
| 239mAm | 2500(200) keV | 163(12) ns | (7/2+) | ||||
| 240Am | 95 | 145 | 240.055300(15) | 50.8(3) h | β+ | 240Pu | (3-) |
| α (1.9×10−4%) | 236Np | ||||||
| 241AmMost common isotopes | 95 | 146 | 241.0568291(20) | 432.2(7) a | α | 237Np | 5/2- |
| CD Cluster decay Cluster decay is a type of nuclear decay in which a parent atomic nucleus with A nucleons and Z protons emits a cluster of Ne neutrons and Ze protons heavier than an alpha particle but lighter than a typical binary fission fragment Cluster decay (also named heavy particle radioactivity or heavy... (7.4×10−10%) |
207Tl, 34Si | ||||||
| SF (4.3×10−10%) | (various) | ||||||
| 241mAm | 2200(100) keV | 1.2(3) µs | |||||
| 242Am | 95 | 147 | 242.0595492(20) | 16.02(2) h | β- (82.7%) | 242Cm | 1- |
| EC (17.3%) | 242Pu | ||||||
| 242m1Am | 48.60(5) keV | 141(2) a | IT Isomeric transition An isomeric transition is a radioactive decay process that involves emission of a gamma ray from an atom where the nucleus is in an excited metastable state, referred to in its excited state, as a nuclear isomer.... (99.54%) |
242Am | 5- | ||
| α (.46%) | 238Np | ||||||
| SF (1.5×10−8%) | (various) | ||||||
| 242m2Am | 2200(80) keV | 14.0(10) ms | (2+,3-) | ||||
| 243Am | 95 | 148 | 243.0613811(25) | 7,370(40) a | α | 239Np | 5/2- |
| SF (3.7×10−9%) | (various) | ||||||
| 244Am | 95 | 149 | 244.0642848(22) | 10.1(1) h | β- | 244Cm | (6-)# |
| 244mAm | 86.1(10) keV | 26(1) min | β- (99.96%) | 244Cm | 1+ | ||
| EC (.0361%) | 244Pu | ||||||
| 245Am | 95 | 150 | 245.066452(4) | 2.05(1) h | β- | 245Cm | (5/2)+ |
| 246Am | 95 | 151 | 246.069775(20) | 39(3) min | β- | 246Cm | (7-) |
| 246m1Am | 30(10) keV | 25.0(2) min | β- (99.99%) | 246Cm | 2(-) | ||
| IT (.01%) | 246Am | ||||||
| 246m2Am | ~2000 keV | 73(10) µs | |||||
| 247Am | 95 | 152 | 247.07209(11)# | 23.0(13) min | β- | 247Cm | (5/2)# |
| 248Am | 95 | 153 | 248.07575(22)# | 3# min | β- | 248Cm | |
| 249Am | 95 | 154 | 249.07848(32)# | 1# min | β- | 249Cm | |

