
Joback method
Encyclopedia
The Joback method predicts
eleven important and commonly used pure component thermodynamic properties from molecular structure only.
 The Joback method is a group contribution method
The Joback method is a group contribution method
. These kind of methods use basic structural information of a chemical molecule like a list of simple functional groups, adds parameters to these functional groups, and calculates thermophysical and transport properties as a function of the sum of group parameters.
Joback assumes that there are no interactions between the groups and therefore only uses additive contributions and no contributions for interactions between groups. Other group contribution methods, especially methods like UNIFAC
, which estimate mixture properties like activity coefficients, use both simple additive group parameters and group interaction parameters. The big advantage of using only simple group parameters is the small number of needed parameters. The number of needed group interaction parameters gets very high for an increasing number of groups (1 for two groups, 3 for three groups, 6 for four groups, 45 for ten groups and twice as much if the interactions are not symmetric.).
Nine of the properties are single temperature-independent values, mostly estimated by a simple sum of group contribution plus an addend.
Two of the estimated properties are temperature-dependent: the ideal gas heat capacity
and the dynamic viscosity
of liquids. The heat capacity polynomial
uses four parameters and the viscosity equation only 2. In both cases the equation parameters are calculated by group contributions.
and uses very similar groups, formulas, and parameters for the three properties the Lydersen already supported (critical temperature, critical pressure, critical volume).
Joback extended the range of supported properties, created new parameters and modified slightly the formulas of the old Lydersen method.
The Joback method additionally uses a very simple and easy to assign group scheme which makes the method usable also for people with only basic chemical knowledge.
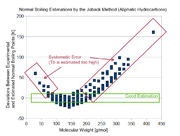 Newer developments of estimation methods have shown that the quality of the Joback method is limited. The original authors already stated themselves in the original paper: “High accuracy is not claimed, but the proposed method are often as or more accurate than techniques in common use today.”.
Newer developments of estimation methods have shown that the quality of the Joback method is limited. The original authors already stated themselves in the original paper: “High accuracy is not claimed, but the proposed method are often as or more accurate than techniques in common use today.”.
The list of groups don't cover many common molecules sufficiently. Especially aromatic compounds are not differentiated from normal ring containing components. This is a severe problem because aromatic and aliphatic components differ strongly.
The data base Joback and Reid used for obtaining the group parameters was rather small and covered only a limited number of different molecules. The best coverage has been achieved for normal boiling points (438 components) and the worst for heat of fusion (155 components). Current developments which can use data banks like the Dortmund Data Bank
or the DIPPR data base have a much broader coverage.
The formula used for the prediction of the normal boiling point shows another problem. Joback assumed a constant contribution of added groups in homologous series like the alkane
s. This doesn't describe the real behavior of the normal boiling points correctly. Instead of the constant contribution a decrease of the contribution with increasing number of groups must be applied. The chosen formula of the Joback method leads to high deviations for large and small molecules and an acceptable good estimation only for mid-sized components.
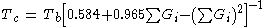
This critical temperature equation needs a normal boiling point Tb. If an experimental value is available it is recommended to use this boiling point. It is, on the other hand, also possible to input the normal boiling point estimated by the Joback method. This will lead to a higher error.
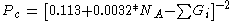
NA: Number of atoms in the molecular structure (including hydrogens).
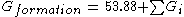
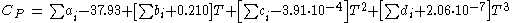
The Joback method uses a four parameter polynomial to describe the temperature dependency of the ideal gas heat capacity. These parameters are valid from 273 K to approx. 1000 K.

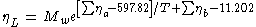
Mw: Molecular Weight
The method uses a two parameter equation to describe the temperature dependency of the dynamic viscosity. The authors state that the parameters are valid from the melting temperature up to 0.7 of the critical temperature (Tr<0.7).
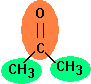
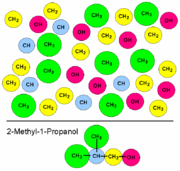
Acetone
(Propanone) is the simplest ketone
and is separated into three groups in the Joback method: two methyl groups
(-CH3) and one ketone group (C=O). Since the methyl group is present twice, its contributions have to be added twice.
Prediction
A prediction or forecast is a statement about the way things will happen in the future, often but not always based on experience or knowledge...
eleven important and commonly used pure component thermodynamic properties from molecular structure only.
Group Contribution Method

Group contribution method
A group contribution method is a technique to estimate and predict thermodynamic and other properties from molecular structures.- Introduction :In today's chemical processes hundreds of thousands of components are used...
. These kind of methods use basic structural information of a chemical molecule like a list of simple functional groups, adds parameters to these functional groups, and calculates thermophysical and transport properties as a function of the sum of group parameters.
Joback assumes that there are no interactions between the groups and therefore only uses additive contributions and no contributions for interactions between groups. Other group contribution methods, especially methods like UNIFAC
UNIFAC
The UNIversal Functional Activity Coefficient method is a semi-empirical system for the prediction of non-electrolyte activity estimation in non-ideal mixtures. UNIFAC uses the functional groups present on the molecules that make up the liquid mixture to calculate activity coefficients...
, which estimate mixture properties like activity coefficients, use both simple additive group parameters and group interaction parameters. The big advantage of using only simple group parameters is the small number of needed parameters. The number of needed group interaction parameters gets very high for an increasing number of groups (1 for two groups, 3 for three groups, 6 for four groups, 45 for ten groups and twice as much if the interactions are not symmetric.).
Nine of the properties are single temperature-independent values, mostly estimated by a simple sum of group contribution plus an addend.
Two of the estimated properties are temperature-dependent: the ideal gas heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
and the dynamic viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
of liquids. The heat capacity polynomial
Polynomial
In mathematics, a polynomial is an expression of finite length constructed from variables and constants, using only the operations of addition, subtraction, multiplication, and non-negative integer exponents...
uses four parameters and the viscosity equation only 2. In both cases the equation parameters are calculated by group contributions.
History
The Joback method is an extension of the Lydersen methodLydersen method
The Lydersen method is a group contribution method for the estimation of critical properties temperature , pressure and volume . The Lydersen method is the prototype for and ancestor of many new models like Joback, Klincewicz,Ambrose,...
and uses very similar groups, formulas, and parameters for the three properties the Lydersen already supported (critical temperature, critical pressure, critical volume).
Joback extended the range of supported properties, created new parameters and modified slightly the formulas of the old Lydersen method.
Strengths
The popularity and success of the Joback method mainly originates from the single group list for all properties. This allows to get all eleven supported properties from a single analysis of the molecular structure.The Joback method additionally uses a very simple and easy to assign group scheme which makes the method usable also for people with only basic chemical knowledge.
Weaknesses

The list of groups don't cover many common molecules sufficiently. Especially aromatic compounds are not differentiated from normal ring containing components. This is a severe problem because aromatic and aliphatic components differ strongly.
The data base Joback and Reid used for obtaining the group parameters was rather small and covered only a limited number of different molecules. The best coverage has been achieved for normal boiling points (438 components) and the worst for heat of fusion (155 components). Current developments which can use data banks like the Dortmund Data Bank
Dortmund Data Bank
The Dortmund Data Bank is a factual data bank for thermodynamic and thermophysical data. Its main usage is the data supply for process simulation where experimental data are the basis for the design, analysis, synthesis, and optimization of chemical processes...
or the DIPPR data base have a much broader coverage.
The formula used for the prediction of the normal boiling point shows another problem. Joback assumed a constant contribution of added groups in homologous series like the alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s. This doesn't describe the real behavior of the normal boiling points correctly. Instead of the constant contribution a decrease of the contribution with increasing number of groups must be applied. The chosen formula of the Joback method leads to high deviations for large and small molecules and an acceptable good estimation only for mid-sized components.
Formulas
In the following formulas Gi denotes a group contribution. Gi are counted for every single available group. If a group is present multiple times each occurrence is counted separately.Critical Temperature
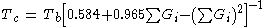
This critical temperature equation needs a normal boiling point Tb. If an experimental value is available it is recommended to use this boiling point. It is, on the other hand, also possible to input the normal boiling point estimated by the Joback method. This will lead to a higher error.
Critical Pressure
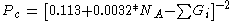
NA: Number of atoms in the molecular structure (including hydrogens).
Gibbs Energy of Formation (Ideal Gas, 298 K)
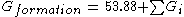
Heat Capacity (Ideal Gas)
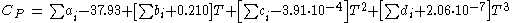
The Joback method uses a four parameter polynomial to describe the temperature dependency of the ideal gas heat capacity. These parameters are valid from 273 K to approx. 1000 K.
Heat of Vaporization at Normal Boiling Point

Liquid Dynamic Viscosity
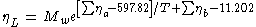
Mw: Molecular Weight
The method uses a two parameter equation to describe the temperature dependency of the dynamic viscosity. The authors state that the parameters are valid from the melting temperature up to 0.7 of the critical temperature (Tr<0.7).
Group Contributions
| Group | Tc | Pc | Vc | Tb | Tm | Hform | Gform | a | b | c | d | Hfusion | Hvap | a | b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Critical State Data | Temperatures of Phase Transitions |
Chemical Caloric Properties |
Ideal Gas Heat Capacities | Enthalpies of Phase Transitions |
Dynamic Viscosity | ||||||||||
| Non-ring groups | |||||||||||||||
| -CH3 | 0.0141 | -0.0012 | 65 | 23.58 | -5.10 | -76.45 | -43.96 | 1.95E+1 | -8.08E-3 | 1.53E-4 | -9.67E-8 | 0.908 | 2.373 | 548.29 | -1.719 |
| -CH2- | 0.0189 | 0.0000 | 56 | 22.88 | 11.27 | -20.64 | 8.42 | -9.09E-1 | 9.50E-2 | -5.44E-5 | 1.19E-8 | 2.590 | 2.226 | 94.16 | -0.199 |
| >CH- | 0.0164 | 0.0020 | 41 | 21.74 | 12.64 | 29.89 | 58.36 | -2.30E+1 | 2.04E-1 | -2.65E-4 | 1.20E-7 | 0.749 | 1.691 | -322.15 | 1.187 |
| |
0.0067 | 0.0043 | 27 | 18.25 | 46.43 | 82.23 | 116.02 | -6.62E+1 | 4.27E-1 | -6.41E-4 | 3.01E-7 | -1.460 | 0.636 | -573.56 | 2.307 |
| |
0.0113 | -0.0028 | 56 | 18.18 | -4.32 | -9.630 | 3.77 | 2.36E+1 | -3.81E-2 | 1.72E-4 | -1.03E-7 | -0.473 | 1.724 | 495.01 | -1.539 |
| |
0.0129 | -0.0006 | 46 | 24.96 | 8.73 | 37.97 | 48.53 | -8.00 | 1.05E-1 | -9.63E-5 | 3.56E-8 | 2.691 | 2.205 | 82.28 | -0.242 |
| |
0.0117 | 0.0011 | 38 | 24.14 | 11.14 | 83.99 | 92.36 | -2.81E+1 | 2.08E-1 | -3.06E-4 | 1.46E-7 | 3.063 | 2.138 | n. a. | n. a. |
| |
0.0026 | 0.0028 | 36 | 26.15 | 17.78 | 142.14 | 136.70 | 2.74E+1 | -5.57E-2 | 1.01E-4 | -5.02E-8 | 4.720 | 2.661 | n. a. | n. a. |
| |
0.0027 | -0.0008 | 46 | 9.20 | -11.18 | 79.30 | 77.71 | 2.45E+1 | -2.71E-2 | 1.11E-4 | -6.78E-8 | 2.322 | 1.155 | n. a. | n. a. |
| |
0.0020 | 0.0016 | 37 | 27.38 | 64.32 | 115.51 | 109.82 | 7.87 | 2.01E-2 | -8.33E-6 | 1.39E-9 | 4.151 | 3.302 | n. a. | n. a. |
| Ring groups | |||||||||||||||
| -CH2- | 0.0100 | 0.0025 | 48 | 27.15 | 7.75 | -26.80 | -3.68 | -6.03 | 8.54E-2 | -8.00E-6 | -1.80E-8 | 0.490 | 2.398 | 307.53 | -0.798 |
| >CH- | 0.0122 | 0.0004 | 38 | 21.78 | 19.88 | 8.67 | 40.99 | -2.05E+1 | 1.62E-1 | -1.60E-4 | 6.24E-8 | 3.243 | 1.942 | -394.29 | 1.251 |
| |
0.0042 | 0.0061 | 27 | 21.32 | 60.15 | 79.72 | 87.88 | -9.09E+1 | 5.57E-1 | -9.00E-4 | 4.69E-7 | -1.373 | 0.644 | n. a. | n. a. |
| |
0.0082 | 0.0011 | 41 | 26.73 | 8.13 | 2.09 | 11.30 | -2.14 | 5.74E-2 | -1.64E-6 | -1.59E-8 | 1.101 | 2.544 | 259.65 | -0.702 |
| |
0.0143 | 0.0008 | 32 | 31.01 | 37.02 | 46.43 | 54.05 | -8.25 | 1.01E-1 | -1.42E-4 | 6.78E-8 | 2.394 | 3.059 | -245.74 | 0.912 |
| Halogen groups | |||||||||||||||
| -F | 0.0111 | -0.0057 | 27 | -0.03 | -15.78 | -251.92 | -247.19 | 2.65E+1 | -9.13E-2 | 1.91E-4 | -1.03E-7 | 1.398 | -0.670 | n. a. | n. a. |
| -Cl | 0.0105 | -0.0049 | 58 | 38.13 | 13.55 | -71.55 | -64.31 | 3.33E+1 | -9.63E-2 | 1.87E-4 | -9.96E-8 | 2.515 | 4.532 | 625.45 | -1.814 |
| -Br | 0.0133 | 0.0057 | 71 | 66.86 | 43.43 | -29.48 | -38.06 | 2.86E+1 | -6.49E-2 | 1.36E-4 | -7.45E-8 | 3.603 | 6.582 | 738.91 | -2.038 |
| -I | 0.0068 | -0.0034 | 97 | 93.84 | 41.69 | 21.06 | 5.74 | 3.21E+1 | -6.41E-2 | 1.26E-4 | -6.87E-8 | 2.724 | 9.520 | 809.55 | -2.224 |
| Oxygen groups | |||||||||||||||
| -OH (alcohol) | 0.0741 | 0.0112 | 28 | 92.88 | 44.45 | -208.04 | -189.20 | 2.57E+1 | -6.91E-2 | 1.77E-4 | -9.88E-8 | 2.406 | 16.826 | 2173.72 | -5.057 |
| -OH (phenol) | 0.0240 | 0.0184 | -25 | 76.34 | 82.83 | -221.65 | -197.37 | -2.81 | 1.11E-1 | -1.16E-4 | 4.94E-8 | 4.490 | 12.499 | 3018.17 | -7.314 |
| -O- (nonring) | 0.0168 | 0.0015 | 18 | 22.42 | 22.23 | -132.22 | -105.00 | 2.55E+1 | -6.32E-2 | 1.11E-4 | -5.48E-8 | 1.188 | 2.410 | 122.09 | -0.386 |
| -O- (ring) | 0.0098 | 0.0048 | 13 | 31.22 | 23.05 | -138.16 | -98.22 | 1.22E+1 | -1.26E-2 | 6.03E-5 | -3.86E-8 | 5.879 | 4.682 | 440.24 | -0.953 |
| >C=O (nonring) | 0.0380 | 0.0031 | 62 | 76.75 | 61.20 | -133.22 | -120.50 | 6.45 | 6.70E-2 | -3.57E-5 | 2.86E-9 | 4.189 | 8.972 | 340.35 | -0.350 |
| >C=O (ring) | 0.0284 | 0.0028 | 55 | 94.97 | 75.97 | -164.50 | -126.27 | 3.04E+1 | -8.29E-2 | 2.36E-4 | -1.31E-7 | 0. | 6.645 | n. a. | n. a. |
| O=CH- (aldehyde) | 0.0379 | 0.0030 | 82 | 72.24 | 36.90 | -162.03 | -143.48 | 3.09E+1 | -3.36E-2 | 1.60E-4 | -9.88E-8 | 3.197 | 9.093 | 740.92 | -1.713 |
| -COOH (acid) | 0.0791 | 0.0077 | 89 | 169.09 | 155.50 | -426.72 | -387.87 | 2.41E+1 | 4.27E-2 | 8.04E-5 | -6.87E-8 | 11.051 | 19.537 | 1317.23 | -2.578 |
| -COO- (ester) | 0.0481 | 0.0005 | 82 | 81.10 | 53.60 | -337.92 | -301.95 | 2.45E+1 | 4.02E-2 | 4.02E-5 | -4.52E-8 | 6.959 | 9.633 | 483.88 | -0.966 |
| |
0.0143 | 0.0101 | 36 | -10.50 | 2.08 | -247.61 | -250.83 | 6.82 | 1.96E-2 | 1.27E-5 | -1.78E-8 | 3.624 | 5.909 | 675.24 | -1.340 |
| Nitrogen groups | |||||||||||||||
| -NH2 | 0.0243 | 0.0109 | 38 | 73.23 | 66.89 | -22.02 | 14.07 | 2.69E+1 | -4.12E-2 | 1.64E-4 | -9.76E-8 | 3.515 | 10.788 | n. a. | n. a. |
| >NH (non-ring) | 0.0295 | 0.0077 | 35 | 50.17 | 52.66 | 53.47 | 89.39 | -1.21 | 7.62E-2 | -4.86E-5 | 1.05E-8 | 5.099 | 6.436 | n. a. | n. a. |
| >NH (ring) | 0.0130 | 0.0114 | 29 | 52.82 | 101.51 | 31.65 | 75.61 | 1.18E+1 | -2.30E-2 | 1.07E-4 | -6.28E-8 | 7.490 | 6.930 | n. a. | n. a. |
| >N-(nonring) | 0.0169 | 0.0074 | 9 | 11.74 | 48.84 | 123.34 | 163.16 | -3.11E+1 | 2.27E-1 | -3.20E-4 | 1.46E-7 | 4.703 | 1.896 | n. a. | n. a. |
| -N= (nonring) | 0.0255 | -0.0099 | n. a. | 74.60 | n. a. | 23.61 | n. a. | n. a. | n. a. | n. a. | n. a. | n. a. | 3.335 | n. a. | n. a. |
| -N= (ring) | 0.0085 | 0.0076 | 34 | 57.55 | 68.40 | 93.70 | 119.66 | 5.69 | -4.12E-3 | 1.28E-4 | -8.88E-8 | 3.649 | 6.528 | n. a. | n. a. |
| |
n. a. | n. a. | n. a. | 83.08 | 68.91 | 93.70 | 119.66 | 5.69 | -4.12E-3 | 1.28E-4 | -8.88E-8 | n. a. | 12.169 | n. a. | n. a. |
| -CN | 0.0496 | -0.0101 | 91 | 125.66 | 59.89 | 88.43 | 89.22 | 3.65E+1 | -7.33E-2 | 1.84E-4 | -1.03E-7 | 2.414 | 12.851 | n. a. | n. a. |
| -NO2 | 0.0437 | 0.0064 | 91 | 152.54 | 127.24 | -66.57 | -16.83 | 2.59E+1 | -3.74E-3 | 1.29E-4 | -8.88E-8 | 9.679 | 16.738 | n. a. | n. a. |
| Sulfur groups | |||||||||||||||
| -SH | 0.0031 | 0.0084 | 63 | 63.56 | 20.09 | -17.33 | -22.99 | 3.53E+1 | -7.58E-2 | 1.85E-4 | -1.03E-7 | 2.360 | 6.884 | n. a. | n. a. |
| -S- (nonring) | 0.0119 | 0.0049 | 54 | 68.78 | 34.40 | 41.87 | 33.12 | 1.96E+1 | -5.61E-3 | 4.02E-5 | -2.76E-8 | 4.130 | 6.817 | n. a. | n. a. |
| -S- (ring) | 0.0019 | 0.0051 | 38 | 52.10 | 79.93 | 39.10 | 27.76 | 1.67E+1 | 4.81E-3 | 2.77E-5 | -2.11E-8 | 1.557 | 5.984 | n. a. | n. a. |
Example Calculation

Acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
(Propanone) is the simplest ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
and is separated into three groups in the Joback method: two methyl groups
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
(-CH3) and one ketone group (C=O). Since the methyl group is present twice, its contributions have to be added twice.
| |
|
||||||
| Property | No. of groups | Group value | No. of groups | Group value | 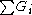 |
Estimated Value | Unit |
| Tc | |||||||
| Pc | |||||||
| Vc | |||||||
| Tb | |||||||
| Tm | |||||||
| Hformation | |||||||
| Gformation | |||||||
| Cpa | |||||||
| Cpb | |||||||
| Cpc | |||||||
| Cpd | |||||||
| Cp | |||||||
| Hfusion | |||||||
| Hvap | |||||||
| ηa | |||||||
| ηb | |||||||
| η | |||||||

