
UNIFAC
Encyclopedia
The UNIversal Functional Activity Coefficient (UNIFAC) method is a semi-empirical
system for the prediction of non-electrolyte activity
estimation in non-ideal mixtures. UNIFAC uses the functional group
s present on the molecules that make up the liquid mixture to calculate activity coefficients. By utilising interactions for each of the functional groups present on the molecules, as well as some binary interaction coefficients, the activity of each of the solutions can be calculated. This information can be used to obtain the liquid equilibria information, which is useful in many thermodynamic calculations, such as chemical reactor
design, and distillation
calculations.
The UNIFAC model was first published in 1975 by Fredenslund, Jones and Prausnitz, a group of chemical engineering researchers from the University of California
. Subsequently they and other authors have published a wide range of UNIFAC papers, extending the capabilities of the model; this has been by the development of new or revision of existing UNIFAC model parameters. UNIFAC is an attempt by these researchers to provide a flexible liquid equilibria model for wider use in chemistry
, the chemical
and process engineering
disciplines.
is the sourcing of reliable thermodynamic constants. These constants are necessary for the successful prediction of the free energy
state of the system; without this information it is impossible to model the equilibrium
phases of the system.
Obtaining this free energy data is not a trivial problem, and requires careful experiments, such as calorimetry
, to successfully measure the energy of the system. Even when this work is performed it is infeasible to attempt to conduct this work for every single possible class of chemicals, and the binary, or higher, mixtures thereof. To alleviate this problem, free energy prediction models, such as UNIFAC, are employed to predict the system's energy based on a few previously measured constants.
Although it is theoretically possible to calculate some of these parameters using ab initio
computer simulations, several major problems with this approach exist; firstly, and most importantly, the computational resources for such calculations are immense - scaling extremely unfavourably for systems with more than a few atoms. Secondly the energies obtained from these calculations obtained from ab initio simulations often require experimental verification to confirm their results. Finally such calculations require a significant level of expertise and a good understanding of quantum chemistry
. Thus the need for simplified models that still successfully predict the thermodynamic state of the system, such as UNIFAC.
of the components in a system is a correction factor that accounts for deviations of real systems from that of an Ideal solution
, which can either be measured via experiment or estimated from chemical models (such as UNIFAC). By adding a correction factor, known as the activity (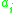 , the activity of the ith component) to the liquid phase fraction of a liquid mixture, some of the effects of the real solution can be accounted for. The activity of a real chemical is a function of the thermodynamic state of the system, i.e. temperature and pressure.
, the activity of the ith component) to the liquid phase fraction of a liquid mixture, some of the effects of the real solution can be accounted for. The activity of a real chemical is a function of the thermodynamic state of the system, i.e. temperature and pressure.
Equipped with the activity coefficients and a knowledge of the constituents and their relative amounts, phenomena such as phase separation and vapour-liquid equilibria can be calculated. UNIFAC attempts to be a general model for the successful prediction of activity coefficients.
 and a residual component
and a residual component 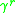 . For the
. For the 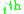 molecule, the activity coefficients are broken down as per the following equation:
molecule, the activity coefficients are broken down as per the following equation:
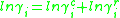
In the UNIFAC model, there are three main parameters required to determine the activity for each molecule in the system. Firstly there are the group surface area and volume contributions
and volume contributions  obtained from the Van der Waals
obtained from the Van der Waals
surface area and volumes. These parameters depend purely upon the individual functional groups on the host molecules. Finally there is the binary interaction parameter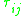 , which is related to the interaction energy
, which is related to the interaction energy 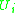 of molecular pairs (equation in "residual" section). These parameters must be obtained either through experiments, via data fitting or molecular simulation.
of molecular pairs (equation in "residual" section). These parameters must be obtained either through experiments, via data fitting or molecular simulation.
model.

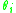 and
and 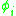 are the molar weighted segment and area fractional components for the
are the molar weighted segment and area fractional components for the 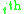 molecule in the total system and are defined by the following equation;
molecule in the total system and are defined by the following equation; 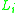 is a compound parameter of
is a compound parameter of  ,
,  and
and 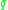 .
.  is the coordination number
is the coordination number
of the system, but the model is found to be relatively insensitive to its value and is frequently quoted as a constant having the value of 10.
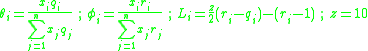
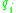 and
and 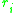 are calculated from the group surface area and volume contributions
are calculated from the group surface area and volume contributions 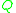 and
and  (Usually obtained via tabulated values) as well as the number of occurrences of the functional group on each molecule
(Usually obtained via tabulated values) as well as the number of occurrences of the functional group on each molecule 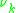 such that:
such that:
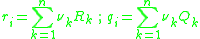
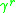 is due to interactions between groups present in the system, with the original paper referring to the concept of a "solution-of-groups". The residual component of the activity for the
is due to interactions between groups present in the system, with the original paper referring to the concept of a "solution-of-groups". The residual component of the activity for the 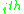 molecule containing
molecule containing  unique functional groups can be written as follows:
unique functional groups can be written as follows:

where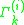 is the activity of an isolated group in a solution consisting only of molecules of type
is the activity of an isolated group in a solution consisting only of molecules of type 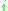 . The formulation of the residual activity ensures that the condition for the limiting case of a single molecule in a pure component solution, the activity is equal to 1; as by the definition of
. The formulation of the residual activity ensures that the condition for the limiting case of a single molecule in a pure component solution, the activity is equal to 1; as by the definition of 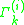 , one finds that
, one finds that 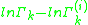 will be zero. The following formula is used for both
will be zero. The following formula is used for both 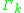 and
and 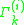
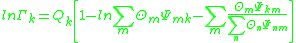
In this formula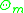 is the summation of the area fraction of group
is the summation of the area fraction of group  , over all the different groups and is somewhat similar in form, but not the same as
, over all the different groups and is somewhat similar in form, but not the same as 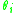 .
. 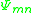 is the group interaction parameter and is a measure of the interaction energy between groups. This is calculated using an Arrhenius equation
is the group interaction parameter and is a measure of the interaction energy between groups. This is calculated using an Arrhenius equation
(albeit with a pesudo-constant of value 1).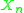 is the group mole fraction, which is the number of groups
is the group mole fraction, which is the number of groups  in the solution divided by the total number of groups.
in the solution divided by the total number of groups.
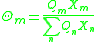
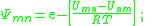
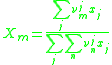
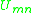 is the energy of interaction between groups m and n, with SI
is the energy of interaction between groups m and n, with SI
units of joules per mole and R is the ideal gas constant. Note that it is not the case that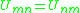 , giving rise to a non-reflexive parameter. The equation for the group interaction parameter can be simplified to the following:
, giving rise to a non-reflexive parameter. The equation for the group interaction parameter can be simplified to the following:
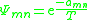
Thus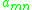 still represents the net energy of interaction between groups
still represents the net energy of interaction between groups  and
and  , but has the somewhat unusual units of absolute temperature (SI kelvin
, but has the somewhat unusual units of absolute temperature (SI kelvin
s). These interaction energy values are obtained from experimental data, and are usually tabulated.
Empirical
The word empirical denotes information gained by means of observation or experimentation. Empirical data are data produced by an experiment or observation....
system for the prediction of non-electrolyte activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
estimation in non-ideal mixtures. UNIFAC uses the functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s present on the molecules that make up the liquid mixture to calculate activity coefficients. By utilising interactions for each of the functional groups present on the molecules, as well as some binary interaction coefficients, the activity of each of the solutions can be calculated. This information can be used to obtain the liquid equilibria information, which is useful in many thermodynamic calculations, such as chemical reactor
Chemical reactor
In chemical engineering, chemical reactors are vessels designed to contain chemical reactions. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction...
design, and distillation
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
calculations.
The UNIFAC model was first published in 1975 by Fredenslund, Jones and Prausnitz, a group of chemical engineering researchers from the University of California
University of California
The University of California is a public university system in the U.S. state of California. Under the California Master Plan for Higher Education, the University of California is a part of the state's three-tier public higher education system, which also includes the California State University...
. Subsequently they and other authors have published a wide range of UNIFAC papers, extending the capabilities of the model; this has been by the development of new or revision of existing UNIFAC model parameters. UNIFAC is an attempt by these researchers to provide a flexible liquid equilibria model for wider use in chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, the chemical
Chemical engineering
Chemical engineering is the branch of engineering that deals with physical science , and life sciences with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms...
and process engineering
Process engineering
Process engineering focuses on the design, operation, control, and optimization of chemical, physical, and biological processes through the aid of systematic computer-based methods...
disciplines.
Introduction
A particular problem in the area of liquid-state thermodynamicsThermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
is the sourcing of reliable thermodynamic constants. These constants are necessary for the successful prediction of the free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
state of the system; without this information it is impossible to model the equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
phases of the system.
Obtaining this free energy data is not a trivial problem, and requires careful experiments, such as calorimetry
Calorimetry
Calorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat...
, to successfully measure the energy of the system. Even when this work is performed it is infeasible to attempt to conduct this work for every single possible class of chemicals, and the binary, or higher, mixtures thereof. To alleviate this problem, free energy prediction models, such as UNIFAC, are employed to predict the system's energy based on a few previously measured constants.
Although it is theoretically possible to calculate some of these parameters using ab initio
Ab initio quantum chemistry methods
Ab initio quantum chemistry methods are computational chemistry methods based on quantum chemistry. The term ab initiowas first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study on the excited states of benzene.The background is described by Parr...
computer simulations, several major problems with this approach exist; firstly, and most importantly, the computational resources for such calculations are immense - scaling extremely unfavourably for systems with more than a few atoms. Secondly the energies obtained from these calculations obtained from ab initio simulations often require experimental verification to confirm their results. Finally such calculations require a significant level of expertise and a good understanding of quantum chemistry
Quantum chemistry
Quantum chemistry is a branch of chemistry whose primary focus is the application of quantum mechanics in physical models and experiments of chemical systems...
. Thus the need for simplified models that still successfully predict the thermodynamic state of the system, such as UNIFAC.
UNIFAC Correlation
The UNIFAC correlation attempts to break down the problem of predicting interactions between molecules by describing molecular interactions based upon the functional groups attached to the molecule. This is done in order to reduce the sheer number of binary interactions that would be needed to be measured to predict the state of the system.Chemical Activity
The activity coefficientActivity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
of the components in a system is a correction factor that accounts for deviations of real systems from that of an Ideal solution
Ideal solution
In chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...
, which can either be measured via experiment or estimated from chemical models (such as UNIFAC). By adding a correction factor, known as the activity (
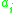 , the activity of the ith component) to the liquid phase fraction of a liquid mixture, some of the effects of the real solution can be accounted for. The activity of a real chemical is a function of the thermodynamic state of the system, i.e. temperature and pressure.
, the activity of the ith component) to the liquid phase fraction of a liquid mixture, some of the effects of the real solution can be accounted for. The activity of a real chemical is a function of the thermodynamic state of the system, i.e. temperature and pressure.Equipped with the activity coefficients and a knowledge of the constituents and their relative amounts, phenomena such as phase separation and vapour-liquid equilibria can be calculated. UNIFAC attempts to be a general model for the successful prediction of activity coefficients.
Model Parameters
The UNIFAC model splits up the activity coefficient for each species in the system into two components; a combinatorial and a residual component
and a residual component 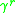 . For the
. For the 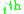 molecule, the activity coefficients are broken down as per the following equation:
molecule, the activity coefficients are broken down as per the following equation: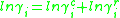
In the UNIFAC model, there are three main parameters required to determine the activity for each molecule in the system. Firstly there are the group surface area
 and volume contributions
and volume contributions  obtained from the Van der Waals
obtained from the Van der WaalsJohannes Diderik van der Waals
Johannes Diderik van der Waals was a Dutch theoretical physicist and thermodynamicist famous for his work on an equation of state for gases and liquids....
surface area and volumes. These parameters depend purely upon the individual functional groups on the host molecules. Finally there is the binary interaction parameter
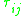 , which is related to the interaction energy
, which is related to the interaction energy 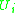 of molecular pairs (equation in "residual" section). These parameters must be obtained either through experiments, via data fitting or molecular simulation.
of molecular pairs (equation in "residual" section). These parameters must be obtained either through experiments, via data fitting or molecular simulation.Combinatorial
The combinatorial component of the activity is contributed to by several terms in its equation (below), and is the same as for the UNIQUACUNIQUAC
UNIQUAC is an activity coefficient model used in description of phase equilibria.The model is a so-called lattice model and has been derived from a first order approximation...
model.

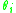 and
and 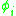 are the molar weighted segment and area fractional components for the
are the molar weighted segment and area fractional components for the 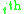 molecule in the total system and are defined by the following equation;
molecule in the total system and are defined by the following equation; 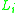 is a compound parameter of
is a compound parameter of  ,
,  and
and 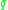 .
.  is the coordination number
is the coordination numberCoordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
of the system, but the model is found to be relatively insensitive to its value and is frequently quoted as a constant having the value of 10.
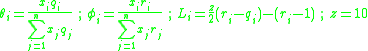
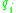 and
and 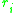 are calculated from the group surface area and volume contributions
are calculated from the group surface area and volume contributions 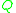 and
and  (Usually obtained via tabulated values) as well as the number of occurrences of the functional group on each molecule
(Usually obtained via tabulated values) as well as the number of occurrences of the functional group on each molecule 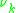 such that:
such that: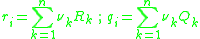
Residual
The residual component of the activity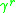 is due to interactions between groups present in the system, with the original paper referring to the concept of a "solution-of-groups". The residual component of the activity for the
is due to interactions between groups present in the system, with the original paper referring to the concept of a "solution-of-groups". The residual component of the activity for the 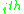 molecule containing
molecule containing  unique functional groups can be written as follows:
unique functional groups can be written as follows:
where
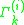 is the activity of an isolated group in a solution consisting only of molecules of type
is the activity of an isolated group in a solution consisting only of molecules of type 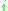 . The formulation of the residual activity ensures that the condition for the limiting case of a single molecule in a pure component solution, the activity is equal to 1; as by the definition of
. The formulation of the residual activity ensures that the condition for the limiting case of a single molecule in a pure component solution, the activity is equal to 1; as by the definition of 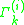 , one finds that
, one finds that 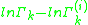 will be zero. The following formula is used for both
will be zero. The following formula is used for both 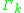 and
and 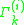
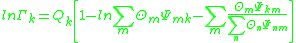
In this formula
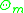 is the summation of the area fraction of group
is the summation of the area fraction of group  , over all the different groups and is somewhat similar in form, but not the same as
, over all the different groups and is somewhat similar in form, but not the same as 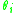 .
. 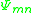 is the group interaction parameter and is a measure of the interaction energy between groups. This is calculated using an Arrhenius equation
is the group interaction parameter and is a measure of the interaction energy between groups. This is calculated using an Arrhenius equationArrhenius equation
The Arrhenius equation is a simple, but remarkably accurate, formula for the temperature dependence of the reaction rate constant, and therefore, rate of a chemical reaction. The equation was first proposed by the Dutch chemist J. H. van 't Hoff in 1884; five years later in 1889, the Swedish...
(albeit with a pesudo-constant of value 1).
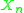 is the group mole fraction, which is the number of groups
is the group mole fraction, which is the number of groups  in the solution divided by the total number of groups.
in the solution divided by the total number of groups.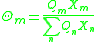
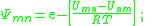
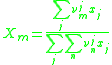
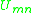 is the energy of interaction between groups m and n, with SI
is the energy of interaction between groups m and n, with SISi
Si, si, or SI may refer to :- Measurement, mathematics and science :* International System of Units , the modern international standard version of the metric system...
units of joules per mole and R is the ideal gas constant. Note that it is not the case that
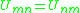 , giving rise to a non-reflexive parameter. The equation for the group interaction parameter can be simplified to the following:
, giving rise to a non-reflexive parameter. The equation for the group interaction parameter can be simplified to the following: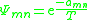
Thus
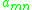 still represents the net energy of interaction between groups
still represents the net energy of interaction between groups  and
and  , but has the somewhat unusual units of absolute temperature (SI kelvin
, but has the somewhat unusual units of absolute temperature (SI kelvinKelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
s). These interaction energy values are obtained from experimental data, and are usually tabulated.
See also
- Chemical equilibriumChemical equilibriumIn a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
- Chemical thermodynamicsChemical thermodynamicsChemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics...
- FugacityFugacityIn chemical thermodynamics, the fugacity of a real gas is an effective pressure which replaces the true mechanical pressure in accurate chemical equilibrium calculations. It is equal to the pressure of an ideal gas which has the same chemical potential as the real gas. For example, nitrogen gas ...
- UNIQUACUNIQUACUNIQUAC is an activity coefficient model used in description of phase equilibria.The model is a so-called lattice model and has been derived from a first order approximation...
- UNIversal QUasi-chemical Activity Coefficients - UNIFAC ConsortiumUNIFAC ConsortiumThe UNIFAC Consortium has been founded at the Carl von Ossietzky University of Oldenburg at the chair of industrial chemistry of Prof. Gmehling to invite private companies to support the further development of the group contribution methods UNIFAC and its successor modified UNIFAC...
- PSRKPSRKPSRK is an estimation method for the calculation of phase equilibria of mixtures of chemical components. The original goal for the development of this method was to enable the estimation of properties of mixtures which contain supercritical components...
- Predictive Soave-Redlich-Kwong - MOSCEDMOSCEDMOSCED is a thermodynamic model for the estimation of limiting activity coefficients . From a historical point of view MOSCED can be regarded as an improved modification of the Hansen method and the Hildebrand solubility model...
- Modified Separation of Cohesive Energy Density Model (Estimation of activity coefficients at infinite dilution)
Further reading
- Vapor-liquid equilibria using UNIFAC : a group contribution methodGroup contribution methodA group contribution method is a technique to estimate and predict thermodynamic and other properties from molecular structures.- Introduction :In today's chemical processes hundreds of thousands of components are used...
, Aage Fredenslund, Jürgen GmehlingJürgen GmehlingJürgen Gmehling is a German professor for technical and industrial chemistry at the Carl von Ossietzky University of Oldenburg.-Biography:...
and Peter Rasmussen,Elsevier Scientific New York, 1979

