Kulinkovich reaction
Encyclopedia
The Kulinkovich reaction describes the organic synthesis
of cyclopropanol
s via reaction of ester
s with dialkyldialkoxytitanium reagents, generated in situ from Grignard reagents bearing hydrogen
in beta-position and titanium(IV) alkoxides such as titanium isopropoxide. This reaction was discovered by Kulinkovich and coworkers in 1989. The titanium reagent can be used catalytically.
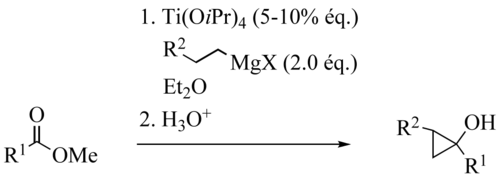 Titanium catalysts are ClTi(OiPr)3 or Ti(OiPr)4, ClTi(OtBu)3 or Ti(OtBu)4, Grignard reagents are EtMgX, PrMgX or BuMgX. Solvents can be Et2O, THF, Toluol. Tolerated Functional Group
Titanium catalysts are ClTi(OiPr)3 or Ti(OiPr)4, ClTi(OtBu)3 or Ti(OtBu)4, Grignard reagents are EtMgX, PrMgX or BuMgX. Solvents can be Et2O, THF, Toluol. Tolerated Functional Group
s: Ether
s R-O-R, R-S-R, Imine
s RN=CHR. Amide
s, primary and secondary amine
s, carbamate
s are not stable to the reaction condition
An asymmetric version of this reaction is also known with a TADDOL-based catalyst.
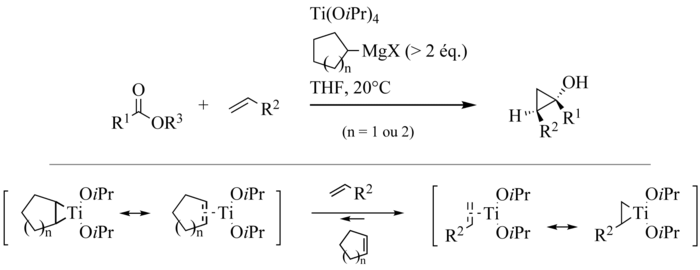
initially utilizes two successive stages of transmetallation of the committed Grignard reagent, leading to an intermediate dialkyldiisopropyloxytitanium complex. This complex undergoes a dismutation to give an alkane molecule and a titanacyclopropane 1. The insertion of the carbonyl
group of the ester
in the weakest carbon-titanium bond leads to an oxatitanacyclopentane 2 being rearranged to ketone
3. Lastly, the insertion of the carbonyl group of 3 in the residual carbon-titanium connection forms a cyclopropane ring. In the transition state
of this elementary stage, which is the limiting stage of the reaction, an agostic interaction
stabilizing between the beta hydrogen and the R2 group and the titanium atom was called upon to explain the diastereoselectivity observed. Complex 4 obtained is a tetraalkyloxytitanium compound able to play a part similar to that of the starting tetraisopropyloxytitanate, which closes the catalytic cycle
. At the end of the reaction, the product is mainly in the shape of the magnesium alcoholate 5, giving the cyclopropanol after hydrolysis by the reaction medium.
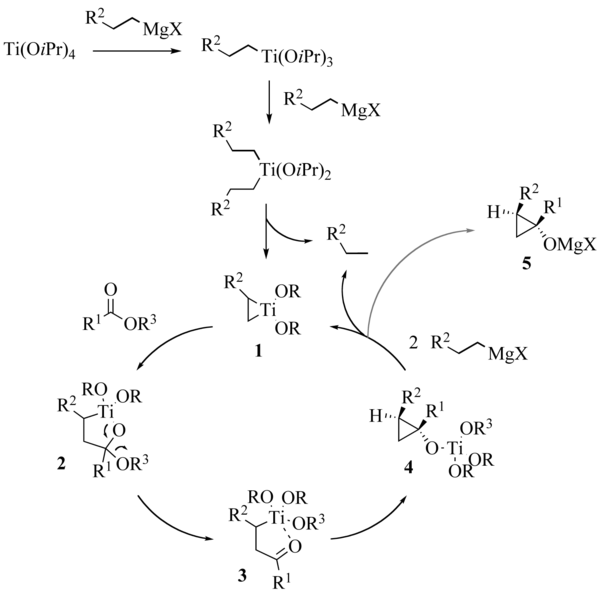 The reaction mechanism of the Kulinkovich reaction was the subject of thorough calculations published in 2001. Certain points remain nevertheless obscure and the mechanism of this reaction is actually not so simple. Intermediate titanium complexes of the ate
The reaction mechanism of the Kulinkovich reaction was the subject of thorough calculations published in 2001. Certain points remain nevertheless obscure and the mechanism of this reaction is actually not so simple. Intermediate titanium complexes of the ate
type were recently proposed by Kulinkovich.
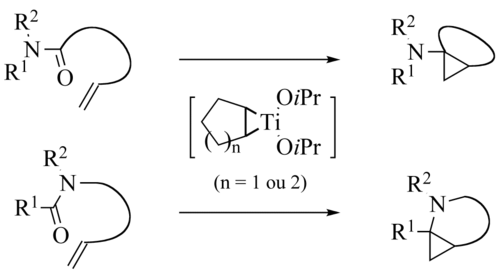
The methodology has been extended to intramolecular
reactions
s instead of esters the reaction product is an aminocyclopropane in the de Meijere variation
The intramolecular reaction is also known :
and the reaction product a cyclopropane with a primary amine
group.
The reaction mechanism is akin the Kulinkovich reaction:
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of cyclopropanol
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
s via reaction of ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s with dialkyldialkoxytitanium reagents, generated in situ from Grignard reagents bearing hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
in beta-position and titanium(IV) alkoxides such as titanium isopropoxide. This reaction was discovered by Kulinkovich and coworkers in 1989. The titanium reagent can be used catalytically.
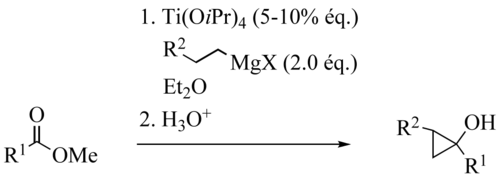
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s: Ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
s R-O-R, R-S-R, Imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s RN=CHR. Amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s, primary and secondary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s, carbamate
Carbamate
Carbamates are organic compounds derived from carbamic acid . A carbamate group, carbamate ester, and carbamic acids are functional groups that are inter-related structurally and often are interconverted chemically. Carbamate esters are also called urethanes.-Synthesis:Carbamic acids are derived...
s are not stable to the reaction condition
An asymmetric version of this reaction is also known with a TADDOL-based catalyst.
Reaction mechanism
The generally accepted reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
initially utilizes two successive stages of transmetallation of the committed Grignard reagent, leading to an intermediate dialkyldiisopropyloxytitanium complex. This complex undergoes a dismutation to give an alkane molecule and a titanacyclopropane 1. The insertion of the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group of the ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
in the weakest carbon-titanium bond leads to an oxatitanacyclopentane 2 being rearranged to ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
3. Lastly, the insertion of the carbonyl group of 3 in the residual carbon-titanium connection forms a cyclopropane ring. In the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
of this elementary stage, which is the limiting stage of the reaction, an agostic interaction
Agostic interaction
Agostic interaction is a term in organometallic chemistry for the interaction of a coordinately-unsaturated transition metal with a C-H bond, when the two electrons involved in the C-H bond enter the empty d-orbital of a transition metal, resulting in a three-center two-electron bond. Many...
stabilizing between the beta hydrogen and the R2 group and the titanium atom was called upon to explain the diastereoselectivity observed. Complex 4 obtained is a tetraalkyloxytitanium compound able to play a part similar to that of the starting tetraisopropyloxytitanate, which closes the catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
. At the end of the reaction, the product is mainly in the shape of the magnesium alcoholate 5, giving the cyclopropanol after hydrolysis by the reaction medium.

Ate complex
An ate complex in chemistry is a salt formed by reaction of a Lewis acid with a base whereby the central atom increases its valence . Often in chemical nomenclature the phrase ate is suffixed to the element in question. For example, the ate complex of a boron compound is called a borate...
type were recently proposed by Kulinkovich.
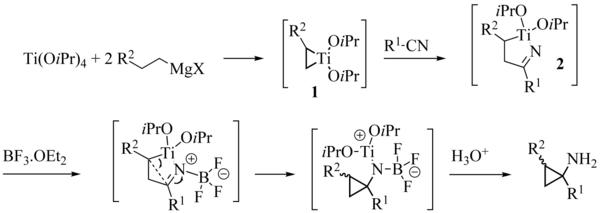
Ligand exchange with olefins
In 1993, the team of Kulinkovich highlighted the aptitude of the titanacyclopropanes to undergo ligand exchange with olefins. This discovery was important, because it gave access to cyclopropanols more functionalized by making economic use of the Grignard of which normally at least two equivalents should have been engaged to obtain good outputs. Cha and its team introduced the use of cyclic Grignard reagents, particularly adapted for these reactions.The methodology has been extended to intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
reactions
de Meijere variation
With amideAmide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
s instead of esters the reaction product is an aminocyclopropane in the de Meijere variation
The intramolecular reaction is also known :
Szymoniak variation
In the Szymoniak variation the substrate is a nitrileNitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
and the reaction product a cyclopropane with a primary amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group.
The reaction mechanism is akin the Kulinkovich reaction: