
Lyman-alpha line
Encyclopedia
In physics
, the Lyman-alpha line, sometimes written as Ly- line, is a spectral line
line, is a spectral line
of hydrogen
, or more generally of one-electron ions
, in the Lyman series
, emitted when the electron
falls from the orbital to the
orbital to the  orbital, where n is the principal quantum number
orbital, where n is the principal quantum number
. In hydrogen, its wavelength
of 1215.668 angstrom
s (1.216×10-7m), corresponding to a frequency
of 2.47 × 1015 hertz, places the Lyman-alpha line in the vacuum ultraviolet part of the electromagnetic spectrum.
Because of fine structure
perturbations, the Lyman-alpha line splits into a doublet. Specifically, because of the electron's spin-orbit interaction
, the stationary eigenstates of the perturbed
Hamiltonian
must be labeled by the total angular momentum j of the electron (spin
plus orbital
), not just the orbital angular momentum
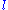 . In the
. In the  orbital, there are two possible states,
orbital, there are two possible states, 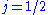 and
and 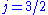 , resulting in a spectral doublet. The
, resulting in a spectral doublet. The 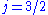 state is of higher energy (less negative) and so is energetically farther from the
state is of higher energy (less negative) and so is energetically farther from the  orbital to which it is transitioning. Thus, the
orbital to which it is transitioning. Thus, the 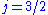 state is associated with the more energetic (shorter wavelength) spectral line in the doublet.
state is associated with the more energetic (shorter wavelength) spectral line in the doublet.
A K-alpha, or Kα, line analogous to the Lyman-alpha line for hydrogen, occurs in the high-energy induced emission spectra of all chemical elements, since it results from the same electron transition as in hydrogen. The equation for prediction of the frequency of this line (usually in the X-ray range for heavier elements), uses the same base-frequency as Lyman-alpha, but modified by a (Z-1)² factor to account for differing atomic numbers (Z) between elements, and is expressed as Moseley's law
. The Lyman-alpha line and the rest of the hydrogen Lyman spectral series are most simply described by the empirical Rydberg equation and semi-classic Bohr model
of the atom.
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, the Lyman-alpha line, sometimes written as Ly-
 line, is a spectral line
line, is a spectral lineSpectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from a deficiency or excess of photons in a narrow frequency range, compared with the nearby frequencies.- Types of line spectra :...
of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, or more generally of one-electron ions
Hydrogen-like atom
A hydrogen-like ion is any atomic nucleus with one electron and thus is isoelectronic with hydrogen. Except for the hydrogen atom itself , these ions carry the positive charge e, where Z is the atomic number of the atom. Examples of hydrogen-like ions are He+, Li2+, Be3+ and B4+...
, in the Lyman series
Lyman series
In physics and chemistry, the Lyman series is the series of transitions and resulting ultraviolet emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1...
, emitted when the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
falls from the
 orbital to the
orbital to the  orbital, where n is the principal quantum number
orbital, where n is the principal quantum numberPrincipal quantum number
In atomic physics, the principal quantum symbolized as n is the firstof a set of quantum numbers of an atomic orbital. The principal quantum number can only have positive integer values...
. In hydrogen, its wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
of 1215.668 angstrom
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
s (1.216×10-7m), corresponding to a frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
of 2.47 × 1015 hertz, places the Lyman-alpha line in the vacuum ultraviolet part of the electromagnetic spectrum.
Because of fine structure
Fine structure
In atomic physics, the fine structure describes the splitting of the spectral lines of atoms due to first order relativistic corrections.The gross structure of line spectra is the line spectra predicted by non-relativistic electrons with no spin. For a hydrogenic atom, the gross structure energy...
perturbations, the Lyman-alpha line splits into a doublet. Specifically, because of the electron's spin-orbit interaction
Spin-orbit interaction
In quantum physics, the spin-orbit interaction is any interaction of a particle's spin with its motion. The first and best known example of this is that spin-orbit interaction causes shifts in an electron's atomic energy levels due to electromagnetic interaction between the electron's spin and...
, the stationary eigenstates of the perturbed
Perturbation theory (quantum mechanics)
In quantum mechanics, perturbation theory is a set of approximation schemes directly related to mathematical perturbation for describing a complicated quantum system in terms of a simpler one. The idea is to start with a simple system for which a mathematical solution is known, and add an...
Hamiltonian
Hamiltonian (quantum mechanics)
In quantum mechanics, the Hamiltonian H, also Ȟ or Ĥ, is the operator corresponding to the total energy of the system. Its spectrum is the set of possible outcomes when one measures the total energy of a system...
must be labeled by the total angular momentum j of the electron (spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
plus orbital
Azimuthal quantum number
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital...
), not just the orbital angular momentum
Azimuthal quantum number
The azimuthal quantum number is a quantum number for an atomic orbital that determines its orbital angular momentum and describes the shape of the orbital...
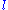 . In the
. In the  orbital, there are two possible states,
orbital, there are two possible states, 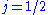 and
and 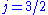 , resulting in a spectral doublet. The
, resulting in a spectral doublet. The 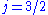 state is of higher energy (less negative) and so is energetically farther from the
state is of higher energy (less negative) and so is energetically farther from the  orbital to which it is transitioning. Thus, the
orbital to which it is transitioning. Thus, the 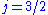 state is associated with the more energetic (shorter wavelength) spectral line in the doublet.
state is associated with the more energetic (shorter wavelength) spectral line in the doublet.A K-alpha, or Kα, line analogous to the Lyman-alpha line for hydrogen, occurs in the high-energy induced emission spectra of all chemical elements, since it results from the same electron transition as in hydrogen. The equation for prediction of the frequency of this line (usually in the X-ray range for heavier elements), uses the same base-frequency as Lyman-alpha, but modified by a (Z-1)² factor to account for differing atomic numbers (Z) between elements, and is expressed as Moseley's law
Moseley's law
Moseley's law is an empirical law concerning the characteristic x-rays that are emitted by atoms. The law was discovered and published by the English physicist Henry Moseley in 1913...
. The Lyman-alpha line and the rest of the hydrogen Lyman spectral series are most simply described by the empirical Rydberg equation and semi-classic Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
of the atom.
See also
- Lyman-alpha forestLyman-alpha forestIn astronomical spectroscopy, the Lyman-alpha forest is the sum of absorption lines arising from the Lyman-alpha transition of the neutral hydrogen in the spectra of distant galaxies and quasars....
- Lyman-alpha emitter
- Lyman-alpha blobLyman-alpha blobIn astronomy, a Lyman-alpha blob is a huge concentration of a gas emitting the Lyman-alpha emission line. LABs are some of the largest known individual objects in the Universe. Some of these gaseous structures are more than 400,000 light years across...
- Moseley's lawMoseley's lawMoseley's law is an empirical law concerning the characteristic x-rays that are emitted by atoms. The law was discovered and published by the English physicist Henry Moseley in 1913...
- Lyman seriesLyman seriesIn physics and chemistry, the Lyman series is the series of transitions and resulting ultraviolet emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1...
- Bohr modelBohr modelIn atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
- Lyman-break galaxyLyman-break galaxyLyman-break galaxies are star-forming galaxies at high redshift that are selected using the differing appearance of the galaxy in several imaging filters due to the position of the Lyman limit...

