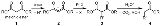
Malonic ester synthesis
Overview
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
where diethyl malonate
Diethyl malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes...
or another ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
of malonic acid
Malonic acid
Malonic acid is a dicarboxylic acid with structure CH22. The ionised form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's ethyl ester...
is alkylated at the carbon alpha
Alpha carbon
The alpha carbon in organic chemistry refers to the first carbon that attaches to a functional group . By extension, the second carbon is the beta carbon, and so on....
(directly adjacent) to both carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups, and then converted to a substituted acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
. The major drawback of malonic ester synthesis is that the alkylation stage can also produce dialkylated structures. This makes separation of products difficult and yields lower.
A strong base is required to deprotonate the center proton.

