
Mukaiyama aldol addition
Encyclopedia
The Mukaiyama aldol addition is an organic reaction
and a type of aldol reaction
between a silyl enol ether
and an aldehyde
catalyzed by a Lewis acid
. This choice of reactants allows for a crossed aldol reaction between an aldehyde
and a ketone
or a different aldehyde without self-condensation of the aldehyde
. For this reason the reaction is used extensively in organic synthesis
. In its original scope the Lewis acid (titanium chloride
) was used in stochiometric amounts but truly catalytic systems exist as well. The reaction is also optimized for asymmetric synthesis.
The archetypical reaction published by Teruaki Mukaiyama in 1973 is that of the silyl enol ether of cyclohexanone
with benzaldehyde
with one equivalent of titanium tetrachloride
in dichloromethane
. At room temperature
it produces a diastereomeric mixture of threo (63%) and erythro (19%) β-hydroxyketone as well as 6% of the exocyclic enone
condensation product
. A subsequent paper in 1974 dealt with the reaction between isopropenyl acetate (the adduct of acetone
and acetic acid
) and benzaldehyde
with various Lewis acids such as aluminium chloride
, tin tetrachloride and boron trifluoride
but many side-reaction competed with formation of the hydroxyketone. Then in another 1974 paper returning to silyl enol ethers the reaction temperature is reduced to -78°C which results in the desired diastereoselectivity.
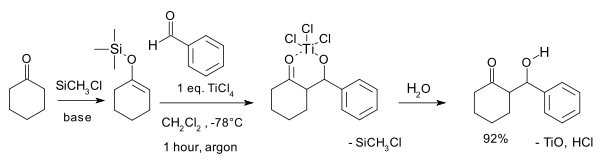 The trimethylsilyl
The trimethylsilyl
group activates the enol
as a nucleophile
and the initial reaction product is a titanium chelate which breaks down on hydrolysis
. The capture of the initial formed aldol product is a prerequisite for the success of the reaction.
A typical reaction involving two ketones is that between acetophenone
as the enol and acetone
:
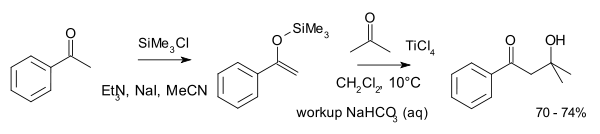 Ketone reactions of this type require higher reaction temperatures.
Ketone reactions of this type require higher reaction temperatures.
For this work Mukaiyama was inspired by earlier work done by Georg Wittig
in 1966 on crossed aldol reactions with lithiated imine
s . Competing work with lithium enolate aldol reactions was published also in 1973 by Herbert O. House
(1999) two aldol additions , one with an ketene silyl acetal and excess magnesium bromide
:
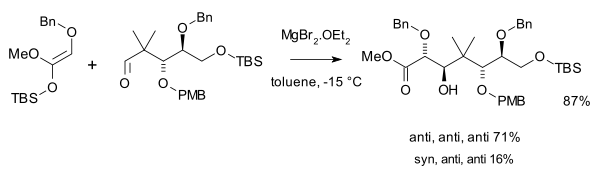 and a second one with an amine chiral ligand
and a second one with an amine chiral ligand
and a triflate salt catalyst:
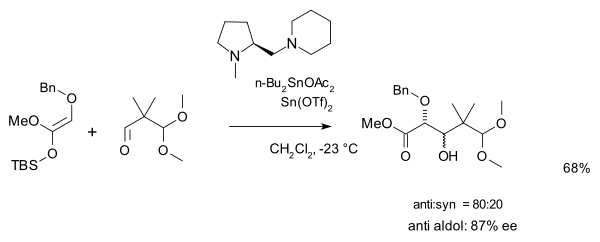
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
and a type of aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
between a silyl enol ether
Silyl enol ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen terminus to an organosilicon group....
and an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
catalyzed by a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
. This choice of reactants allows for a crossed aldol reaction between an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
and a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
or a different aldehyde without self-condensation of the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
. For this reason the reaction is used extensively in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. In its original scope the Lewis acid (titanium chloride
Titanium chloride
Titanium chloride may refer to:* Titanium tetrachloride , TiCl4* Titanium trichloride , TiCl3* Titanium dichloride , TiCl2...
) was used in stochiometric amounts but truly catalytic systems exist as well. The reaction is also optimized for asymmetric synthesis.
The archetypical reaction published by Teruaki Mukaiyama in 1973 is that of the silyl enol ether of cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
with benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
with one equivalent of titanium tetrachloride
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
in dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
. At room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
it produces a diastereomeric mixture of threo (63%) and erythro (19%) β-hydroxyketone as well as 6% of the exocyclic enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
condensation product
Aldol condensation
An aldol condensation is an organic reaction in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone....
. A subsequent paper in 1974 dealt with the reaction between isopropenyl acetate (the adduct of acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
and acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
) and benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
with various Lewis acids such as aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
, tin tetrachloride and boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
but many side-reaction competed with formation of the hydroxyketone. Then in another 1974 paper returning to silyl enol ethers the reaction temperature is reduced to -78°C which results in the desired diastereoselectivity.

Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
group activates the enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
as a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
and the initial reaction product is a titanium chelate which breaks down on hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
. The capture of the initial formed aldol product is a prerequisite for the success of the reaction.
A typical reaction involving two ketones is that between acetophenone
Acetophenone
Acetophenone is the organic compound with the formula C6H5CCH3. It is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.-Production:Acetophenone can be obtained by a variety of methods...
as the enol and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
:

For this work Mukaiyama was inspired by earlier work done by Georg Wittig
Georg Wittig
Georg Wittig was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Chemistry with Herbert C...
in 1966 on crossed aldol reactions with lithiated imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
s . Competing work with lithium enolate aldol reactions was published also in 1973 by Herbert O. House
Scope
Mukaiyama employed in his rendition of taxol total synthesisTaxol total synthesis
Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel . This diterpenoid is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the Pacific yew...
(1999) two aldol additions , one with an ketene silyl acetal and excess magnesium bromide
Magnesium bromide
Magnesium bromide is a chemical compound of magnesium and bromine that is white and deliquescent. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. It is water soluble and somewhat soluble in alcohol...
:

Chiral ligand
In chemistry a chiral ligand is a specially adapted ligand used for asymmetric synthesis. This ligand is an enantiopure organic compound which combines with a metal center by chelation to form an asymmetric catalyst. This catalyst engages in a chemical reaction and transfers its chirality to the...
and a triflate salt catalyst:


