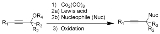
Nicholas reaction
Encyclopedia
The Nicholas reaction is an organic reaction
where a dicobalt octacarbonyl
-stabilized propargyl
ic cation is reacted with a nucleophile
. Oxidative demetallation gives the desired alkylated alkyne
.
 Several reviews have been published.
Several reviews have been published.
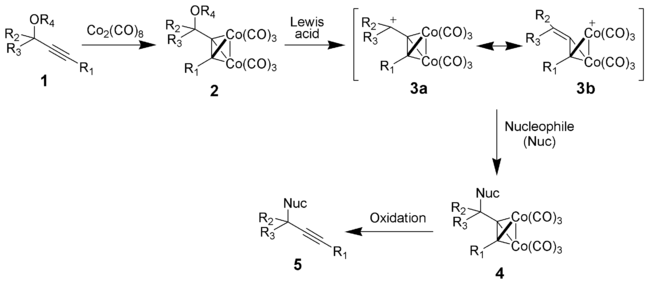 The addition of dicobalt octacarbonyl
The addition of dicobalt octacarbonyl
to a propargylic ether
(1) gives the dicobalt intermediate 2. Reaction with HBF4 or Lewis acid
gives the key dicobalt octacarbonyl-stabilized propargyl
ic cation (3a and 3b). Addition of a nucleophile
followed by a mild oxidation gives the desired substituted alkyne (5).
The likely intermediates
in the reactions, [(propargylium)Co2(CO)6]+ cation 3, possessed considerable stability. It was, in fact, possible to observe these cations by 1H NMR
at 10°C when generated using d-trifluoroacetic acid
. Later, Connor and Nicholas were able to isolate salts of such cations 3 as stable, dark red solids by treatment of the Co2(CO)6-complexed propargyl alcohols with excess fluoroantimonic acid
or tetrafluoroboric acid etherate. The reason that these complexes are so remarkably stable is due to significant delocalization of the cationic charge onto the Co2(CO)6 moiety.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
where a dicobalt octacarbonyl
Dicobalt octacarbonyl
Dicobalt octacarbonyl is the inorganic compound Co28. This metal carbonyl is a reagent and catalyst in organometallic chemistry and organic synthesis. It is used as a catalyst for hydroformylation, the conversion of alkenes to aldehydes....
-stabilized propargyl
Propargyl
In organic chemistry, propargyl is an alkyl functional group of 2-propynyl with the structure HC≡C−CH2−, derived from the alkyne propyne.The term propargylic refers to a saturated position on a molecular framework next to an alkynyl group...
ic cation is reacted with a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. Oxidative demetallation gives the desired alkylated alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
.

Reaction mechanism

Dicobalt octacarbonyl
Dicobalt octacarbonyl is the inorganic compound Co28. This metal carbonyl is a reagent and catalyst in organometallic chemistry and organic synthesis. It is used as a catalyst for hydroformylation, the conversion of alkenes to aldehydes....
to a propargylic ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
(1) gives the dicobalt intermediate 2. Reaction with HBF4 or Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
gives the key dicobalt octacarbonyl-stabilized propargyl
Propargyl
In organic chemistry, propargyl is an alkyl functional group of 2-propynyl with the structure HC≡C−CH2−, derived from the alkyne propyne.The term propargylic refers to a saturated position on a molecular framework next to an alkynyl group...
ic cation (3a and 3b). Addition of a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
followed by a mild oxidation gives the desired substituted alkyne (5).
The likely intermediates
Reaction intermediate
A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants and reacts further to give the directly observed products of a chemical reaction. Most chemical reactions are stepwise, that is they take more than one elementary step to complete...
in the reactions, [(propargylium)Co2(CO)6]+ cation 3, possessed considerable stability. It was, in fact, possible to observe these cations by 1H NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
at 10°C when generated using d-trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
. Later, Connor and Nicholas were able to isolate salts of such cations 3 as stable, dark red solids by treatment of the Co2(CO)6-complexed propargyl alcohols with excess fluoroantimonic acid
Fluoroantimonic acid
Fluoroantimonic acid is a mixture of hydrogen fluoride and antimony pentafluoride in various ratios. The 1:1 combination forms the strongest known superacid, which has been demonstrated to protonate even hydrocarbons to afford carbocations and H2....
or tetrafluoroboric acid etherate. The reason that these complexes are so remarkably stable is due to significant delocalization of the cationic charge onto the Co2(CO)6 moiety.

