Organocopper compound
Encyclopedia
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
contain carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
to copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s. Organocopper chemistry is the science of organocopper compounds describing their physical properties, synthesis and reactions. They are reagents in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
.
Brief history
The first organocopper compound, the explosive copper(I) acetylideCopper(I) acetylide
Copper acetylide, or cuprous acetylide, is an inorganic chemical compound with the formula Cu2C2. It is a heat and shock sensitive high explosive, more sensitive than silver acetylide. It is a metal acetylide. It is similar to silver acetylide and calcium carbide, though it is not called carbide in...
Cu2C2 (Cu-C≡C-Cu), was synthesized by Rudolf Christian Böttger
Rudolf Christian Böttger
Rudolf Christian Böttger was a German inorganic chemist. He conducted most of his research at the University of Frankfurt am Main...
in 1859 by passing acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
gas through copper(I) chloride
Copper(I) chloride
Copper chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid...
solution:
- C2H2 + 2 CuCl → Cu2C2 + 2 HCl
Henry Gilman
Henry Gilman
Henry Gilman was an American organic chemist known as the father of organometallic chemistry, the field within which his most notable work was done. He discovered the Gilman reagent, which bears his name....
prepared methylcopper in 1936. In 1941, Kharash discovered that reaction of a Grignard reagent with cyclohexenone in presence of Cu(I) resulted in 1,4-addition instead of 1,2-addition. In 1952 Gilman investigated for the first time dialkylcuprates. In the 1960s, complexes of alkenes and CO with copper(I) were established.
Properties
Organocopper compounds are very reactive towards oxygen and water forming copper(I) oxideCopper(I) oxide
Copper oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper. This red-coloured solid is a component of some antifouling paints. The compound can appear either yellow or red, depending on the size of the particles, but both forms...
and tend to be thermally unstable. Because most cuprates are salts, many are generally insoluble in nonpolar solvents. Despite these difficulties, organocopper reagents are frequently generated and consumed in situ
In situ
In situ is a Latin phrase which translated literally as 'In position'. It is used in many different contexts.-Aerospace:In the aerospace industry, equipment on board aircraft must be tested in situ, or in place, to confirm everything functions properly as a system. Individually, each piece may...
with no attempt to isolate them. They are used very frequently in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
as alkylating reagents because they exhibit more functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
tolerance than corresponding Grignard and organolithium reagents. The electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of copper is much higher than its next-door neighbour in the group 12 element
Group 12 element
A group 12 element is one of the elements in group 12 in the periodic table. This includes zinc , cadmium and mercury . The further inclusion of copernicium in group 12 is supported by recent experiments on individual Cn atoms...
s, zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, suggesting less nucleophilicity for its carbon ligands.
Copper belongs to the group of coinage metals together with silver and gold and their chemistries have many similarities. The oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
can be +1 or +2 and intermediates can have oxidation state +3. Monovalent alkylcopper compounds (RCu) are polymeric but form cuprate
Cuprate
Cuprates are chemical compounds containing copper anion. Cuprates have been known for centuries and are widely used in inorganic and organic chemistry...
s (R2CuLi) upon treatment with organolithium compounds (RLi). These cuprates are sometimes referred to as Gilman reagent
Gilman reagent
A Gilman reagent is a lithium and copper reagent compound, R2CuLi, where R is an organic radical. These are useful because they react with organic chlorides, bromides, and iodides to replace the halide group with an R group. This is extremely useful in creating larger molecules from smaller...
s. Organocopper compounds can be stabilized by complexation to a variety of ligands such as organophosphanes (R3P), thioethers (R2S), and cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
(CN-).
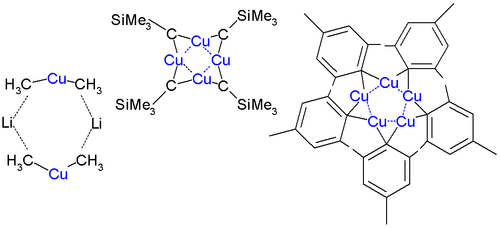
The cuprates complex form complicated aggregates both in crystalline form and in solution. Lithium dimethylcuprate is a dimer in diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
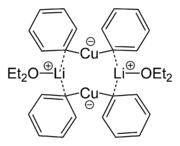
forming an 8-membered ring with two lithium atoms coordinating between two methyl groups. Similarly, lithium diphenylcuprate forms a dimeric etherate, [{Li(OEt2)}(CuPh2)]2, in the solid state.
The first ever crystal structure was determined in 1972 by Lappert for CuCH2SiMe3. This compound is relatively stable because the bulky trimethylsilyl
Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
groups provide steric protection. It is a tetramer forming an 8-membered ring with alternating Cu-C bonds. In addition the four copper atoms form a planar Cu4 ring based on three-center two-electron bond
Three-center two-electron bond
A three-center two-electron bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one non-bonding, and one anti-bonding. The two electrons go into the bonding orbital, resulting in a...
s. The copper to copper bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
is 242 pm compared to 256 pm in bulk copper. In pentamesitylpentacopper a 5-membered copper ring is formed and pentafluorophenylcopper is a tetramer.
With carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
copper forms a non-classical metal carbonyl.
Cu(III) intermediates
In many organometallic reactions involving copper, the reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
invokes a copper intermediate with oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
+3. For instance, in reductive elimination processes, Cu(III) is reduced to Cu(I). However Cu(III) compounds are rare in chemistry in general and until recently organocopper(III) species have been elusive. In 2007 the first spectroscopic evidence was obtained for the involvement of Cu(III) in the conjugate addition of the Gilman reagent
Gilman reagent
A Gilman reagent is a lithium and copper reagent compound, R2CuLi, where R is an organic radical. These are useful because they react with organic chlorides, bromides, and iodides to replace the halide group with an R group. This is extremely useful in creating larger molecules from smaller...
to an enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
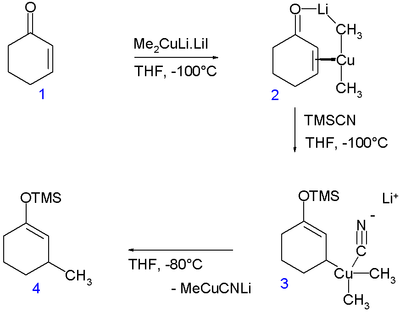
: In a so-called rapid-injection NMR
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
experiment at -100°C, the Gilman reagent Me2CuLi (stabilized by lithium iodide
Lithium iodide
Lithium iodide, or LiI, is a compound of lithium and iodine. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine.-Applications:...
) was introduced to cyclohexenone
Cyclohexenone
Cyclohexenone is an organic compound which is a versatile intermediate used in the synthesis of a variety of chemical products such as pharmaceuticals and fragrances...
(1) enabling the detection of the copper — alkene pi complex 2. On subsequent addition of trimethylsilyl cyanide
Trimethylsilyl cyanide
Trimethylsilyl cyanide is the chemical compound with the formula 3SiCN. This volatile liquid consists of a cyanide group, that is CN, attached to a trimethylsilyl group. The molecule is used in organic synthesis as the equivalent of hydrogen cyanide...
the Cu(III) species 3 is formed (indefinitely stable at that temperature) and on increasing the temperature to -80°C the conjugate addition product 4. According to an accompanying in silico
In silico
In silico is an expression used to mean "performed on computer or via computer simulation." The phrase was coined in 1989 as an analogy to the Latin phrases in vivo and in vitro which are commonly used in biology and refer to experiments done in living organisms and outside of living organisms,...
experiments the Cu(III) intermediate has a square planar molecular geometry with the cyano group in cis orientation with respect to the cyclohexenyl methine
Methine
In chemistry, methine is a trivalent functional group CH, derived formally from methane. The methine group consists of a carbon atom bound by two single bonds and one double bond, where one of the single bonds is to a hydrogen...
group and anti-parallel to the methine proton. With other ligands than the cyano group this study predicts room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
stable Cu(III) compounds.
Synthesis
Copper halides react with organolithium reagentOrganolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s to form the organocopper compound. Phenylcopper is prepared by reaction of phenyllithium
Phenyllithium
Phenyllithium is an organometallic agent with the empirical formula C6H5Li. It is most commonly used as a metalating agent in organic syntheses and a substitute for Grignard reagents for introducing phenyl groups in organic syntheses...
with copper(I) bromide
Copper(I) bromide
Copper bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds....
in diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
. Reaction with a second equivalent of R-Li to R-Cu then gives the lithium diorganocopper compound. Copper halides also react with Grignard reagents. The compound pentamesitylpentacopper is prepared from mesityl magnesium bromide and copper(I) chloride
Copper(I) chloride
Copper chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid...
.
Copper salts add to terminal alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
s to form copper acetylides The copper metallocene
Metallocene
A metallocene is a compound typically consisting of two cyclopentadienyl anions bound to a metal center in the oxidation state II, with the resulting general formula 2M. Closely related to the metallocenes are the metallocene derivatives, e.g. titanocene dichloride, vanadocene dichloride...
(η-cyclopentadienyl triethylphosphine) copper can be prepared by reaction of copper(II) oxide
Copper(II) oxide
Copper oxide or cupric oxide is the higher oxide of copper. As a mineral, it is known as tenorite.-Chemistry:It is a black solid with an ionic structure which melts above 1200 °C with some loss of oxygen...
with cyclopentadiene
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
and triethylphosphine in pentane
Pentane
Pentane is an organic compound with the formula C5H12 — that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two being called...
at reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
.
Substitution reactions
Substitution reactionSubstitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
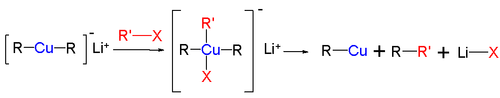
s of cuprates R2CuLi to alkyl halides R'-X gives the alkylcopper compound R-Cu, the coupling product R-R', and the lithium halide Li-X. The reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is based on nucleophilic attack, namely oxidative addition
Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre...
of the alkyl halide to Cu(I) elevating it to a planar
Trigonal planar
In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of a triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands are identical and all bond angles are 120°. Such species belong to...
Cu(III) intermediate followed by reductive elimination. The nucleophilic attack is the rate-determining step. In the case for substitution of iodide, single electron transfer mechanism is proposed (see figure).
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s > tosylates ~ epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s > iodides > bromides > chlorides > ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s > ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s > nitrile
Nitrile
A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called...
s >> alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s
Coupling reactions
Oxidative coupling is the coupling of copper acetylidesMetal acetylide
Acetylide, ethynide, dicarbide, or percarbide is the divalent anion with formula C22− or 2−. It may be regarded as the result of removing two protons from acetylene C2H2 or H-C≡C-H, the prototypical alkyne — that behaves as a weak acid.These terms are also used for any monovalent anion...
to conjugated alkynes in the Glaser coupling (for example in the synthesis of cyclooctadecanonaene
Cyclooctadecanonaene
Cyclooctadecanonaene or [18]annulene is an annulene with chemical formula . This hydrocarbon obeys Hückel's rule and is, therefore, an aromatic compound...
) or to aryl halides in the Castro-Stephens Coupling
Castro-Stephens coupling
The Castro-Stephens Coupling is a cross coupling reaction between a copper acetylide and an aryl halide forming a disubstituted alkyne and a copper halide....
Reductive coupling is a coupling reaction
Coupling reaction
A coupling reaction in organic chemistry is a catch-all term for a variety of reactions where two hydrocarbon fragments are coupled with the aid of a metal catalyst...
of aryl halides with a stoichiometric equivalent of copper metal that occurs in the Ullmann reaction
Ullmann reaction
The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides with copper. The reaction is named after Fritz Ullmann....
. In an example of a present-day cross-coupling reaction called decarboxylative coupling, a catalytic amount of Cu(I) displaces a carboxyl group forming the arylcopper (ArCu) intermediate. Simultaneously, a palladium catalyst converts an aryl bromide to the organopalladium intermediate (Ar'PdBr), and on transmetallation the biaryl is formed from ArPdAr'.
Redox neutral coupling is the coupling of terminal alkynes with halo-alkynes with a copper(I) salt in the Cadiot-Chodkiewicz coupling
Cadiot-Chodkiewicz coupling
The Cadiot-Chodkiewicz coupling in organic chemistry is a coupling reaction between a terminal alkyne and a haloalkyne catalyzed by a copper salt such as copper bromide and an amine base. The reaction product is a di-acetylene or di-alkyne....
. Thermal coupling of two organocopper compounds is also possible.
Conjugate addition
Conjugate additions to enoneEnone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
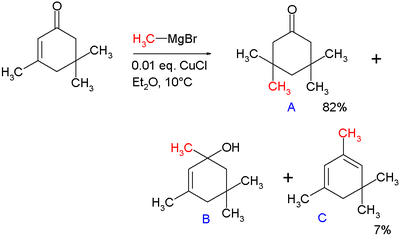
s are done with organocuprates. Note that if a Grignard reagent (such as RMgBr) is used, the reaction with an enone would instead proceed through a 1,2-addition. The 1,4-addition mechanism of cuprates to enones goes through the nucleophilic addition of the Cu(I) species at the beta-carbon of the alkene to form a Cu(III) intermediate, followed by reductive elimination of Cu(I). In the original paper describing this reaction, methylmagnesium bromide is reacted with isophorone
Isophorone
Isophorone is an α,β-Unsaturated cyclic ketone, a colorless to yellowish liquid with characteristic smell, that is used as a solvent and as an intermediate in organic synthesis...
with and without 1 mole percent of added copper chloride
Copper chloride
Copper forms two stable chlorides:*Copper chloride , CuCl, mineral name nantokite.*Copper chloride , CuCl2, mineral name eriochalcite....
(see figure).
Without added salt the main products are alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
B (42%) from nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
to the carbonyl group and diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
C (48%) as its dehydration reaction
Dehydration reaction
In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
product. With added salt the main product is 1,4-adduct A (82%) with some C (7%).
A 1,6-addition is also possible, for example in one step of the commercial-scale production of fulvestrant
Fulvestrant
Fulvestrant, also known as ICI 182,780, is a drug treatment of hormone receptor-positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy. It is an estrogen receptor antagonist with no agonist effects, which works both by down-regulating and...
:
Carbocupration
Carbocupration is a nucleophilic additionNucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
of organocopper reagents (R-Cu) to acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
or terminal alkynes resulting in an alkenylcopper compound (RC=C-Cu). It is a special case of carbometalation
Carbometalation
Carbometalation is an organometallic reaction involving the nucleophilic addition to alkenes and alkynes of a diverse range of organometallic reagents such as organolithium compounds, organocopper compounds and Grignard reagents according to the following general alkyne scheme:The addition can...
and also called the Normant reaction.
See also
- Chemistries of carbon with other elements of the periodic table: