Organogermanium compound
Encyclopedia

Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
to germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
or hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
to germanium chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
. Organogermanium chemistry is the corresponding chemical science. Germanium shares group 14
Carbon group
The carbon group is a periodic table group consisting of carbon , silicon , germanium , tin , lead , and ununquadium ....
in the periodic table with silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
and lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
and not surprisingly the chemistry of organogermanium is in between that of organosilicon compounds
Organosilicon
Organosilicon compounds are organic compounds containing carbon silicon bonds. Organosilicon chemistry is the corresponding science exploring their properties and reactivity.Like carbon, the organically bound silicon is tetravalent and tetrahedral...
and organotin compounds.
The main reason why the organogermanium is of limited synthetic
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
value are the costs of germanium compounds. On the other hand germanium is advocated as a non-toxic alternative to many toxic organotin reagents and compounds like tetramethylgermanium and tetraethylgermanium
Tetraethylgermanium
Tetraethylgermanium , abbreviated TEG, is an organogermanium compound with the formula 4Ge. Tetraethylgermanium is an important chemical compound used in vapour deposition of germanium.-Synthesis:...
are used in the microelectronics industry as precursors for germanium dioxide
Germanium dioxide
Germanium dioxide, also called germanium oxide and germania, is an inorganic compound, an oxide of germanium. Its chemical formula is GeO2. Other names include germanic acid, G-15, and ACC10380...
chemical vapor deposition
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
.
The first organogermanium compound, tetraethylgermane, was synthesised by Winkler in 1887, by the reaction of germanium tetrachloride with diethylzinc
Diethylzinc
Diethylzinc 2Zn, or DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry and available commercially as a solution in hexanes, heptane, or toluene.-Synthesis:Edward Frankland first...
. The organogermanium compound bis (2-Carboxyethylgermanium)sesquioxide was first reported in 1966.
Organogermanes
Organogermanes of the type R4Ge with alkyl (R) groups are accessed through the cheapest available germanium precursor germanium tetrachlorideGermanium tetrachloride
Germanium tetrachloride is a colourless liquid used as an intermediate in the production of purified germanium metal. In recent years, GeCl4 usage has increased substantially due to its use as a reagent for fiber optic production.-Production:...
and alkyl nucleophiles. The following trends are observed going down the carbon group: The nucleophilicity increases Si
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
effect known as the beta-silicon effect
Beta-silicon effect
The beta-silicon effect also called silicon hyperconjugation in organosilicon chemistry is a special type of hyperconjugation and describes the stabilizing effect of a silicon atom placed in a position one removed from a carbocation. A prerequisite is an antiperiplanar relationship between the two...
Si
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
and the Sn-C bond is mainly radical, bonds with germanium are in between.
Just as with silicon the nucleophilicity of allyl germanes is high due to the intrinsic polarization of the bond (difference in electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
2.55 − 2.01 = 0.54) and the combined stabilizing effect on the α-carbonion by the allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
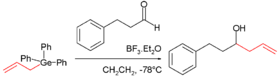
group and the germanium atom. The germanium pendant of the Sakurai reaction
Sakurai reaction
The Sakurai reaction is the chemical reaction of carbon electrophiles with allylic silanes catalyzed by strong Lewis acids. It is named after the chemists Akira Hosomi and Hideki Sakurai.Lewis acid activation is essential for complete reaction...
was discovered in 1986:
The carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group in this reaction is activated with boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
.
Germanium hydrides
Isobutylgermane (IBGe) (Me2CHCH2)GeH3 is the organogermanium hydride that is a high vapor pressure liquid germanium source for MOVPE. Isobutylgermane is currently investigated as safer and less hazardous alternative to toxic germaneGermane
Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and...
gas in microelectonic applications.
Tris(trimethylsilyl)germanium hydride (Me3Si)3GeH has been investigated as a non-toxic alternative to many tin hydrides such as tributyltinhydride
Tributyltin
Tributyltin compounds are a group of compounds containing the 3Sn moiety, such as tributyltin hydride or tributyltin oxide. They are the main active ingredients in certain biocides used to control a broad spectrum of organisms...
.
Other germanium compounds
Many germanium reactive intermediateReactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
s are known: germylenes (carbene
Carbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
pendants), germyl free radicals, germynes (carbyne
Carbyne
In chemistry, a carbyne is a monovalent carbon radical species containing an electrically neutral univalent carbon atom with three non-bonded electrons.- Gas phase/reactive intermediate :...
pendants).
As with silicon and contrasting with carbon, compounds containing Ge-C (germenes) and Ge-Ge (digermylenes) double bonds are unstable but known for instance the benzene pendant germanabenzene
Germanabenzene
Germabenzene is the parent representative of a group of chemical compounds containing in their molecular structure a benzene ring with a carbon atom replaced by a germanium atom. Germabenzene itself has been studied theoretically, but has not been synthesized...
.
External links
- Tetramethylgermanium Datasheet commercial supplier
- Tetraethylgermanium Datasheet commercial supplier
- Tris(trimethylsilyl)germanium hydride Datasheet commercial supplier
See also
- Compounds of carbon with other elements in the periodic table: