Organosulfur compounds
Encyclopedia
Organosulfur compounds are organic compound
s that contain sulfur
. They are often associated with foul odours, but many of the sweetest compounds known are organosulfur derivatives. Nature abounds with organosulfur compounds—sulfur is essential for life. Two of the 20 common amino acids are organosulfur compounds. Fossil fuels, coal
, petroleum
, and natural gas
, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
Sulfur shares the chalcogen
group with oxygen, and it is expected that organosulfur compounds have similarities with carbon-oxygen compounds, which is true to some extent.
A classical chemical test
for the detection of sulfur compounds is the Carius halogen method
.
s Relative to C–C bonds, C–S bond are both longer, because S is larger than carbon, and about 10% weaker. Representative bond length
s in sulfur compounds are 183 pm for the S-C single bond in methanethiol
and 173 pm in thiophene
. The C–S bond dissociation energy
for thiomethane is 89 kcal/mol (370 kJ/mol) compared to methane's 100 kcal/mol (420 kJ/mol) and when hydrogen is replaced by a methyl group the energy decreases to 73 kcal/mol (305 kJ/mol). The single carbon to oxygen bond is shorter than that of the C–C bond. The bond dissociation energies
for dimethyl sulfide
and dimethyl ether
are respectively 73 and 77 kcal/mol (305 and 322 kJ/mol.
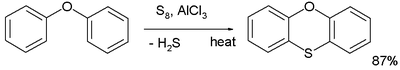
Thioethers are typically prepared by alkylation of thiols. They can also be prepared via the Pummerer rearrangement
. In one named reaction called the Ferrario reaction phenyl ether is converted to phenoxathiin by action of elemental sulfur and aluminium chloride
Thioacetal
s and thioketal
s feature C–S–C–S–C bond sequence. They represent a subclass of thioethers. The thioacetals are useful in "umpolung
" of carbonyl groups.
Thioester
s have general structure R–CO–S–R. They are related to regular esters but are more reactive.
Thiophenes represent a special class of thioethers that are aromatic. The resonance stabilization of thiophene
is 29 kcal/mol (121 kJ/mol) compared to 20 kcal/mol (84 kJ/mol) for the oxygen analogue furan
. The reason for this difference is the higher electronegativity
for oxygen drawing away electrons to itself at the expense of the aromatic ring current. Yet as an aromatic substituent
the thio group is less effective as an activating group
than the alkoxy group.
group contain the functionality R–SH. Thiols are structurally similar to the alcohol
group, but these functionalities are very different in their chemical properties. Thiols are more nucleophilic
, more acidic, and more readily oxidized. This acidity can differ by 5 pKa
units.
The difference in electronegativity
between sulfur (2.58) and hydrogen (2.20) is small and therefore hydrogen bonding in thiols is not prominent. Aliphatic thiols form monolayer
s on gold
, which are topical in nanotechnology
.
Certain aromatic thiols can be accessed through a Herz reaction
.
Disulfides
R–S–S–R with a covalent sulfur to sulfur bond are important for crosslinking: in biochemistry
for the folding and stability of some proteins and in polymer chemistry
for the crosslinking of rubber.
Longer sulfur chains are also known, such as in the natural product varacin
which contains an unusual pentathiepin ring (5-sulfur chain cyclised onto a benzene ring)
s between carbon and sulfur are relatively uncommon, but include important compounds carbon disulfide
, carbonyl sulfide
, and thiophosgene
. Thioketone
s (RC(=S)R') are uncommon with alkyl substituents, but one example example is thiobenzophenone
. Thioaldehydes are rarer still, reflecting their lack of steric protection ("thioformaldehyde" exists as a cyclic trimer). Thioamide
s, with the formula R1C(=S)N(R2)R3 are more common. They are typically prepared by the reaction of amides with Lawesson's reagent
.
Compounds that contain C=S double bonds exist in sulfonium ylides for instance in the Johnson-Corey-Chaykovsky reaction
.
(CS) and have been suggested for the compounds F3CCSF3 and F5SCSF3 . The compound HCSOH is also presented as having a formal triple bond .
s (RC(O)SH)) and dithiocarboxylic acids (RC(S)SH) are well known. They are structurally similar to carboxylic acids but more acidic. Thioamides are analogous to amides.
s have functionality RS(=O)2OH. They are strong acids that are typically soluble in organic solvents. Sulfonic acids like trifluoromethanesulfonic acid
is a frequently used reagent in organic chemistry
. Sulfa drugs are sulfonamide
s derived from aromatic sulfonation
.
group, for instance the compound hexamethylpertellurane (Te(Me)6) was discovered in 1990 by reaction of tetramethyltellurium with xenon difluoride
to Te(Me)2)F2 followed by reaction with diethylzinc
. The sulfur analogue hexamethylpersulfurane SMe6 has been predicted to be stable but has not been synthesized yet.
The first ever all-carbon persulfurane actually synthesized in a laboratory has two methyl and two biphenyl
ligand
s :
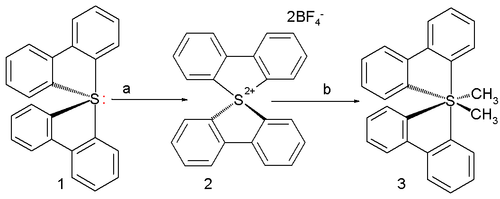 It is prepared from the corresponding sulfurane 1 with xenon difluoride
It is prepared from the corresponding sulfurane 1 with xenon difluoride
/ boron trifluoride
in acetonitrile
to the sulfuranyl dication 2 followed by reaction with methyllithium in tetrahydrofuran
to (a stable) persulfurane 3 as the cis isomer. X-ray diffraction shows C-S bond length
s ranging between 189 and 193 pm (longer than the standard bond length) with the central sulfur atom in a distorted octahedral molecular geometry
.
Computer simulation suggests that these bonds are very polar with the negative charges residing on carbon.
are responsible for the odor of garlic
, and lenthionine
contributes to the flavor of shiitake mushrooms. Many of these natural products also have important medicinal properties such as preventing platelet
aggregation or fighting cancer.
Some organosulfur compounds in the environment, are generated as minor by-products of industrial processes such as the manufacture of plastics and tires.
Selected smell-producing processes are organosulfur compounds produced by the coking of coal designed to drive out sulfurus compounds and other volatile impurities in order to produce 'clean carbon' (coke
), which is primarily used for steel production.
or crude oil into precursor chemicals (feedstocks) for downstream industrial uses (e.g. plastics or pharmaceutical production) and the ubiquitous needs of petroleum distillation for (gasoline
s, diesel, and other grades of fuel oil
s production.
Organosulfur compounds might be understood as smelly contaminants that need to be removed from natural gas before commercial uses, from exhaust stacks and exhaust vents before discharge. In this latter context, organosulfur compounds may be said to account for the pollutants in sulfurous acid rain
, or equivalently, said to be pollutants within most common fossil fuels, especially coal
.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s that contain sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. They are often associated with foul odours, but many of the sweetest compounds known are organosulfur derivatives. Nature abounds with organosulfur compounds—sulfur is essential for life. Two of the 20 common amino acids are organosulfur compounds. Fossil fuels, coal
Coal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
, petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
, and natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
Sulfur shares the chalcogen
Chalcogen
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family...
group with oxygen, and it is expected that organosulfur compounds have similarities with carbon-oxygen compounds, which is true to some extent.
A classical chemical test
Chemical test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to prove the existence of, or to quantify, a chemical compound or chemical group with the aid of a specific reagent...
for the detection of sulfur compounds is the Carius halogen method
Carius halogen method
The Carius halogen method in analytical chemistry is a method for the quantitative determination of halogens in chemical substances .In this technique a chemical substance is oxidized with fuming nitric acid under pressure in the presence of silver nitrate at a maximum operating temperature of 230...
.
Classes of organosulfur compounds
Organosulfur compounds can be classified according to the sulfur-containing functional groups, which are listing in decreasing order of their occurrence.Thioethers, thioesters, thioacetals
These compounds are characterized by C–S–C bondChemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s Relative to C–C bonds, C–S bond are both longer, because S is larger than carbon, and about 10% weaker. Representative bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s in sulfur compounds are 183 pm for the S-C single bond in methanethiol
Methanethiol
Methanethiol is a colorless gas with a smell like rotten cabbage. It is a natural substance found in the blood and brain of humans and other animal as well as plant tissues. It is disposed of through animal feces. It occurs naturally in certain foods, such as some nuts and cheese...
and 173 pm in thiophene
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene...
. The C–S bond dissociation energy
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
for thiomethane is 89 kcal/mol (370 kJ/mol) compared to methane's 100 kcal/mol (420 kJ/mol) and when hydrogen is replaced by a methyl group the energy decreases to 73 kcal/mol (305 kJ/mol). The single carbon to oxygen bond is shorter than that of the C–C bond. The bond dissociation energies
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
for dimethyl sulfide
Dimethyl sulfide
Dimethyl sulfide or methylthiomethane is an organosulfur compound with the formula 2S. Dimethyl sulfide is a water-insoluble flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize,...
and dimethyl ether
Dimethyl ether
Dimethyl ether , also known as methoxymethane, is the organic compound with the formula . The simplest ether, it is a colourless gas that is a useful precursor to other organic compounds and an aerosol propellant. When combusted, DME produces minimal soot and CO, though HC and NOx formation is...
are respectively 73 and 77 kcal/mol (305 and 322 kJ/mol.
Thioethers are typically prepared by alkylation of thiols. They can also be prepared via the Pummerer rearrangement
Pummerer rearrangement
The Pummerer rearrangement is an organic reaction whereby an alkyl sulfoxide rearranges to an α-acyloxy–thioether in the presence of acetic anhydride. In this reaction, sulfur is reduced while adjacent carbon is oxidized....
. In one named reaction called the Ferrario reaction phenyl ether is converted to phenoxathiin by action of elemental sulfur and aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
Thioacetal
Thioacetal
Thioacetals are the sulfur analogue of acetals. They are prepared in a similar way to acetals: by reacting a thiol with an aldehyde:Dithioacetals are prepared similarly to thioacetals, which are intermediates:...
s and thioketal
Thioketal
In chemistry, a thioketal is the sulfur analogue of a ketal, with one of the oxygen replaced by sulfur. A dithioketal has both oxygens replaced by sulfur.Thioketals can be obtained by reacting ketones or aldehydes with thioles....
s feature C–S–C–S–C bond sequence. They represent a subclass of thioethers. The thioacetals are useful in "umpolung
Umpolung
Umpolung or polarity inversion in organic chemistry is the chemical modification of a functional group with the aim of the reversal of polarity of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by...
" of carbonyl groups.
Thioester
Thioester
Thioesters are compounds with the functional group C-S-CO-C. They are the product of esterification between a carboxylic acid and a thiol. Thioesters are widespread in biochemistry, the best-known derivative being acetyl-CoA.-Synthesis:...
s have general structure R–CO–S–R. They are related to regular esters but are more reactive.
Thiophenes represent a special class of thioethers that are aromatic. The resonance stabilization of thiophene
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene...
is 29 kcal/mol (121 kJ/mol) compared to 20 kcal/mol (84 kJ/mol) for the oxygen analogue furan
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans....
. The reason for this difference is the higher electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
for oxygen drawing away electrons to itself at the expense of the aromatic ring current. Yet as an aromatic substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
the thio group is less effective as an activating group
Activating group
In organic chemistry, a functional group is called an activating group if a benzene molecule to which it is attached more readily participates in electrophilic substitution reactions...
than the alkoxy group.
Thiols, disulfides, polysulfides
ThiolThiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
group contain the functionality R–SH. Thiols are structurally similar to the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
group, but these functionalities are very different in their chemical properties. Thiols are more nucleophilic
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
, more acidic, and more readily oxidized. This acidity can differ by 5 pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
units.
The difference in electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
between sulfur (2.58) and hydrogen (2.20) is small and therefore hydrogen bonding in thiols is not prominent. Aliphatic thiols form monolayer
Monolayer
- Chemistry :A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail...
s on gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
, which are topical in nanotechnology
Nanotechnology
Nanotechnology is the study of manipulating matter on an atomic and molecular scale. Generally, nanotechnology deals with developing materials, devices, or other structures possessing at least one dimension sized from 1 to 100 nanometres...
.
Certain aromatic thiols can be accessed through a Herz reaction
Herz reaction
The Herz-reaction, named after the chemist Richard Herz, is the chemical conversion of an aniline-derivative to a so-called Herz-salt with disulfur dichloride, followed by hydrolysis of this Herz-salt to the corresponding sodium thiolate :-Benzothiazoles:The sodium thiolate 3 can be converted to...
.
Disulfides
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
R–S–S–R with a covalent sulfur to sulfur bond are important for crosslinking: in biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
for the folding and stability of some proteins and in polymer chemistry
Polymer chemistry
Polymer chemistry or macromolecular chemistry is a multidisciplinary science that deals with the chemical synthesis and chemical properties of polymers or macromolecules. According to IUPAC recommendations, macromolecules refer to the individual molecular chains and are the domain of chemistry...
for the crosslinking of rubber.
Longer sulfur chains are also known, such as in the natural product varacin
Varacin
Varacin is a bicyclic organosulfur compound originally found in marine ascidiacea from the Polycitor family. It contains an unusual pentathiepin ring which reacts with DNA, and varacin and synthetic analogues have been investigated for their antimicrobial and anti-tumour properties....
which contains an unusual pentathiepin ring (5-sulfur chain cyclised onto a benzene ring)
Thioketones, thioaldehydes, and related compounds
Compounds with double bondDouble bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s between carbon and sulfur are relatively uncommon, but include important compounds carbon disulfide
Carbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
, carbonyl sulfide
Carbonyl sulfide
Carbonyl sulfide is the chemical compound with the formula OCS. Commonly written as COS, it is a colourless flammable gas with an unpleasant odor. It is a linear molecule consisting of a carbonyl group double bonded to a sulfur atom...
, and thiophosgene
Thiophosgene
Thiophosgene is a red liquid with the formula CSCl2. It is a molecule with trigonal planar geometry. There are two reactive C–Cl bonds that allow it to be used in diverse organic syntheses.-Synthesis of CSCl2:...
. Thioketone
Thioketone
Thioketones are organosulfur compounds related to conventional ketones. Instead of the formula R2C=O, thioketones have the formula R2C=S. Unhindered alkylthioketones are typically unstable; such compounds tend to form polymers or rings.-Preparative methods:Thiones are usually prepared from ketones...
s (RC(=S)R') are uncommon with alkyl substituents, but one example example is thiobenzophenone
Thiobenzophenone
Thiobenzophenone is an organosulfur compound with the formula 2CS. It is the prototypical thioketone. Unlike other thioketones that tend to dimerize to form rings and polymers, thiobenzophenone is quite stable, although it photoxidizes in air to form benzophenone and sulfur...
. Thioaldehydes are rarer still, reflecting their lack of steric protection ("thioformaldehyde" exists as a cyclic trimer). Thioamide
Thioamide
Thioamide is a functional group with the general structure R-CS-NR'R, where R, R', and R are organic groups. They are analogous to amides but they exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier...
s, with the formula R1C(=S)N(R2)R3 are more common. They are typically prepared by the reaction of amides with Lawesson's reagent
Lawesson's reagent
Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with...
.
Compounds that contain C=S double bonds exist in sulfonium ylides for instance in the Johnson-Corey-Chaykovsky reaction
Johnson-Corey-Chaykovsky reaction
The Johnson–Corey–Chaykovsky reaction is a chemical reaction used in organic chemistry for the synthesis of epoxides, aziridines, and cyclopropanes. It was discovered in 1961 by A. William Johnson and developed significantly by E.J. Corey and Michael Chaykovsky...
.
Triple bonds between carbon and sulfur
Triple bonds between sulfur and carbon in sulfaalkynes are rare and can be found in carbon monosulfideCarbon monosulfide
Carbon monosulfide is a chemical compound with the formula CS. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interstellar medium. The molecule resembles carbon monoxide...
(CS) and have been suggested for the compounds F3CCSF3 and F5SCSF3 . The compound HCSOH is also presented as having a formal triple bond .
Thiocarboxylic acids and thioamides
Thiocarboxylic acidThiocarboxylic acid
Thiocarboxylic acids are organosulfur compounds with the general formula RCSH. They are related to carboxylic acids by replacement of one oxygen centre by sulfur. Two tautomers are possible, written as RCOH and RCSH, but only the latter is observed. In the laboratory, the most common...
s (RC(O)SH)) and dithiocarboxylic acids (RC(S)SH) are well known. They are structurally similar to carboxylic acids but more acidic. Thioamides are analogous to amides.
Sulfonic acids, esters, amides
Sulfonic acidSulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
s have functionality RS(=O)2OH. They are strong acids that are typically soluble in organic solvents. Sulfonic acids like trifluoromethanesulfonic acid
Trifluoromethanesulfonic acid
Trifluoromethanesulfonic acid, also known as triflic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest acids. Triflic acid is mainly used in research as a catalyst for esterification.-Properties:Triflic acid is a hygroscopic, colorless...
is a frequently used reagent in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. Sulfa drugs are sulfonamide
Sulfonamide (chemistry)
In chemistry, the sulfonamide functional group is -S2-NH2, a sulfonyl group connected to an amine group.A sulfonamide is a compound that contains this group. The general formula is RSO2NH2, where R is some organic group. For example, "methanesulfonamide" is CH3SO2NH2...
s derived from aromatic sulfonation
Aromatic sulfonation
Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution.-Mechanism:...
.
Sulfuranes and persulfuranes
Sulfuranes are relatively specialized functional group that are tetravalent, hypervalent sulfur compounds, with the formula SR4 and likewise persulfuranes are hexavalent SR6. All-carbon persulfuranes have been known for the heavier representatives of the chalcogenChalcogen
The chalcogens are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family...
group, for instance the compound hexamethylpertellurane (Te(Me)6) was discovered in 1990 by reaction of tetramethyltellurium with xenon difluoride
Xenon difluoride
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture sensitive. It decomposes on contact with light or water vapour. Xenon difluoride is a dense, white crystalline solid. It...
to Te(Me)2)F2 followed by reaction with diethylzinc
Diethylzinc
Diethylzinc 2Zn, or DEZn, is a highly pyrophoric organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry and available commercially as a solution in hexanes, heptane, or toluene.-Synthesis:Edward Frankland first...
. The sulfur analogue hexamethylpersulfurane SMe6 has been predicted to be stable but has not been synthesized yet.
The first ever all-carbon persulfurane actually synthesized in a laboratory has two methyl and two biphenyl
Biphenyl
Biphenyl is an organic compound that forms colorless crystals. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula 2...
ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s :

Xenon difluoride
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture sensitive. It decomposes on contact with light or water vapour. Xenon difluoride is a dense, white crystalline solid. It...
/ boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
in acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
to the sulfuranyl dication 2 followed by reaction with methyllithium in tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
to (a stable) persulfurane 3 as the cis isomer. X-ray diffraction shows C-S bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s ranging between 189 and 193 pm (longer than the standard bond length) with the central sulfur atom in a distorted octahedral molecular geometry
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
.
Computer simulation suggests that these bonds are very polar with the negative charges residing on carbon.
Naturally occurring organosulfur compounds
Not all organosulfur compounds are foul-smelling pollutants. Compounds like allicin and ajoeneAjoene
Ajoene is a chemical compound available from garlic . The name is derived from "ajo", the Spanish word for garlic. It is found as a mixture of two isomers, E-, and Z- 4,5,9-trithiadodeca-1,6,11-triene 9-oxide....
are responsible for the odor of garlic
Garlic
Allium sativum, commonly known as garlic, is a species in the onion genus, Allium. Its close relatives include the onion, shallot, leek, chive, and rakkyo. Dating back over 6,000 years, garlic is native to central Asia, and has long been a staple in the Mediterranean region, as well as a frequent...
, and lenthionine
Lenthionine
Lenthionine is a cyclic organosulfur compound found in shiitake mushrooms and partly responsible for their flavor. The mechanism of its formation is unclear, but it probably involves the enzyme C-S lyase....
contributes to the flavor of shiitake mushrooms. Many of these natural products also have important medicinal properties such as preventing platelet
Platelet
Platelets, or thrombocytes , are small,irregularly shaped clear cell fragments , 2–3 µm in diameter, which are derived from fragmentation of precursor megakaryocytes. The average lifespan of a platelet is normally just 5 to 9 days...
aggregation or fighting cancer.
Organosulfur compounds in pollution
Most organic sulfur compounds in the environment are naturally occurring, as a consequence of the fact that sulfur is essential for life and two amino acids contain this element.Some organosulfur compounds in the environment, are generated as minor by-products of industrial processes such as the manufacture of plastics and tires.
Selected smell-producing processes are organosulfur compounds produced by the coking of coal designed to drive out sulfurus compounds and other volatile impurities in order to produce 'clean carbon' (coke
Coke (fuel)
Coke is the solid carbonaceous material derived from destructive distillation of low-ash, low-sulfur bituminous coal. Cokes from coal are grey, hard, and porous. While coke can be formed naturally, the commonly used form is man-made.- History :...
), which is primarily used for steel production.
Organosulfur compounds in fossil fuels
Odours occur as well in chemical processing of coalCoal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
or crude oil into precursor chemicals (feedstocks) for downstream industrial uses (e.g. plastics or pharmaceutical production) and the ubiquitous needs of petroleum distillation for (gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
s, diesel, and other grades of fuel oil
Fuel oil
Fuel oil is a fraction obtained from petroleum distillation, either as a distillate or a residue. Broadly speaking, fuel oil is any liquid petroleum product that is burned in a furnace or boiler for the generation of heat or used in an engine for the generation of power, except oils having a flash...
s production.
Organosulfur compounds might be understood as smelly contaminants that need to be removed from natural gas before commercial uses, from exhaust stacks and exhaust vents before discharge. In this latter context, organosulfur compounds may be said to account for the pollutants in sulfurous acid rain
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions . It can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of carbon dioxide, sulfur dioxide and nitrogen...
, or equivalently, said to be pollutants within most common fossil fuels, especially coal
Coal
Coal is a combustible black or brownish-black sedimentary rock usually occurring in rock strata in layers or veins called coal beds or coal seams. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure...
.
External links
- Organosulfur chemistry at http://users.ox.ac.uk Link