
Photochromism
Encyclopedia

Weizmann Institute of Science
The Weizmann Institute of Science , known as Machon Weizmann, is a university and research institute in Rehovot, Israel. It differs from other Israeli universities in that it offers only graduate and post-graduate studies in the sciences....
in Israel
Israel
The State of Israel is a parliamentary republic located in the Middle East, along the eastern shore of the Mediterranean Sea...
proposed the term "photochromism". Photochromism can take place in both organic and inorganic compounds, and also has its place in biological systems (for example retinal
Retinal
Retinal, also called retinaldehyde or vitamin A aldehyde, is one of the many forms of vitamin A . Retinal is a polyene chromophore, and bound to proteins called opsins, is the chemical basis of animal vision...
in the vision process).
Overview
Photochromism does not have a rigorous definition, but is usually used to describe compounds that undergo a reversible photochemical reaction where an absorption bandAbsorption band
An absorption band is a range of wavelengths, frequencies or energies in the electromagnetic spectrum which are able to excite a particular transition in a substance...
in the visible part of the electromagnetic spectrum
Electromagnetic spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The "electromagnetic spectrum" of an object is the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object....
changes dramatically in strength or wavelength. In many cases, an absorbance band is present in only one form. The degree of change required for a photochemical reaction to be dubbed "photochromic" is that which appears dramatic by eye, but in essence there is no dividing line between photochromic reactions and other photochemistry. Therefore, while the trans-cis isomerization of azobenzene
Azobenzene
Azobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
is considered a photochromic reaction, the analogous reaction of stilbene
Stilbene
-Stilbene, is a diarylethene, i.e., a hydrocarbon consisting of a trans ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining....
is not. Since photochromism is just a special case of a photochemical reaction, almost any photochemical reaction type may be used to produce photochromism with appropriate molecular design. Some of the most common processes involved in photochromism are pericyclic reaction
Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
s, cis-trans isomerizations, intramolecular hydrogen transfer, intramolecular group transfers, dissociation
Dissociation
Dissociation is an altered state of consciousness characterized by partial or complete disruption of the normal integration of a person’s normal conscious or psychological functioning. Dissociation is most commonly experienced as a subjective perception of one's consciousness being detached from...
processes and electron transfer
Electron transfer
Electron transfer is the process by which an electron moves from an atom or a chemical species to another atom or chemical species...
s (oxidation-reduction).
Another requirement of photochromism is two states of the molecule should be thermally stable under ambient conditions for a reasonable time. All the same, nitrospiropyran (which back-isomerizes in the dark over ~10 minutes at room temperature) is considered photochromic. All photochromic molecules back-isomerize to their more stable form at some rate, and this back-isomerization is accelerated by heating. There is therefore a close relationship between photochromic and thermochromic compounds. The timescale of thermal back-isomerization is important for applications, and may be molecularly engineered. Photochromic compounds considered to be "thermally stable" include some diarylethenes, which do not back isomerize even after heating at 80 C for 3 months.
Since photochromic chromophores are dyes, and operate according to well-known reactions, their molecular engineering to fine-tune their properties can be achieved relatively easily using known design models, quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
calculations, and experimentation. In particular, the tuning of absorbance bands to particular parts of the spectrum and the engineering of thermal stability have received much attention.
Sometimes, and particularly in the dye industry, the term "irreversible photochromic" is used to describe materials that undergo a permanent color change upon exposure to ultraviolet or visible light radiation. Because by definition photochromics are reversible, there is technically no such thing as an "irreversible photochromic"—this is loose usage, and these compounds are better referred to as "photochangable" or "photoreactive" dyes.
Apart from the qualities already mentioned, several other properties of photochromics are important for their use. These include
- Quantum yieldQuantum yieldThe quantum yield of a radiation-induced process is the number of times that a defined event occurs per photon absorbed by the system. The "event" may represent a chemical reaction, for example the decomposition of a reactant molecule:...
of the photochemical reaction. This determined the efficiency of the photochromic change with respect to the amount of light absorbed. The quantum yield of isomerization can be strongly dependent on conditions (see below). - Fatigue resistance. In photochromic materials, fatigue refers to the loss of reversibility by processes such as photodegradation, photobleaching, photooxidation, and other side reactions. All photochromics suffer fatigue to some extent, and its rate is strongly dependent on the activating light and the conditions of the sample.
- Photostationary statePhotostationary stateThe photostationary state of a reversible photochemical reaction is the equilibrium chemical composition under a specific kind of electromagnetic irradiation . It is a property of particular importance in photochromic compounds, often used as a measaure of their practical efficiency and usually...
. Photochromic materials have two states, and their interconversion can be controlled using different wavelengths of light. Excitation with any given wavelength of light will result in a mixture of the two states at a particular ratio, called the "photostationary state". In a perfect system, there would exist wavelengths that can be used to provide 1:0 and 0:1 ratios of the isomers, but in real systems this is not possible, since the active absorbance bands always overlap to some extent. - Polarity and solubilitySolubilitySolubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
. In order to incorporate photochromics in working systems, they suffer the same issues as other dyes. They are often charged in one or more state, leading to very high polarity and possible large changes in polarity. They also often contain large conjugated systems that limit their solubility.
Photochromic complexes
A photochromic complex is a kind of chemical compound that has photoresponsive parts on its ligandLigand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
. These complexes have a specific structure: photoswitchable organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s are attached to metal complexes. For the photocontrollable parts, thermally and photochemically stable chromophores (azobenzene
Azobenzene
Azobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
, diarylethene
Diarylethene
In chemistry, diarylethene is the general name of a class of compounds that have aromatic groups bonded to each end of a carbon-carbon double bond...
, spiropyran, etc.) are usually used. And for the metal complexes, a wide variety of compounds that have various functions (redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
response, luminescence
Luminescence
Luminescence is emission of light by a substance not resulting from heat; it is thus a form of cold body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions, or stress on a crystal. This distinguishes luminescence from incandescence, which is light emitted by a...
, magnetism
Magnetism
Magnetism is a property of materials that respond at an atomic or subatomic level to an applied magnetic field. Ferromagnetism is the strongest and most familiar type of magnetism. It is responsible for the behavior of permanent magnets, which produce their own persistent magnetic fields, as well...
, etc.) are applied.
The photochromic parts and metal parts are so close that they can affect each other's molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s. The physical properties of these compounds shown by parts of them (i.e., chromophores or metals) thus can be controlled by switching their other sites by external stimuli. For example, photoisomerization behaviors of some complexes can be switched by oxidation and reduction of their metal parts. Some other compounds can be changed in their luminescence behavior, magnetic interaction of metal sites, or stability of metal-to-ligand coordination by photoisomerization of their photochromic parts.
Classes of photochromic materials
Photochromic molecules can belong to various classes: triarylmethanes, stilbeneStilbene
-Stilbene, is a diarylethene, i.e., a hydrocarbon consisting of a trans ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining....
s, azastilbenes, nitrone
Nitrone
A nitrone is the N-oxide of an imine and a functional group in organic chemistry. The general structure is R1R2C=NR3+O- where R3 is different from H.A nitrone is 1,3-dipole in 1,3-dipolar cycloadditions...
s, fulgides, spiropyrans, naphthopyrans, spiro-oxazines, quinones and others.
Spiropyrans and spirooxazines
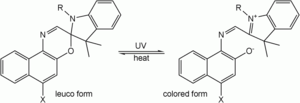
Leuco dye
A leuco dye is a dye whose molecules can acquire two forms, one of which is colorless.For example, the spiro form of an oxazine is a colorless leuco dye; the conjugated system of the oxazine and another aromatic part of the molecule is separated by an sp3-hybridized "spiro" carbon...
; the conjugated system
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
of the oxazine and another aromatic part of the molecule is separated by a sp³-hybridized "spiro" carbon. After irradiation with UV light, the bond between the spiro-carbon and the oxazine breaks, the ring opens, the spiro carbon achieves sp² hybridization and becomes planar, the aromatic group rotates, aligns its π-orbitals with the rest of the molecule, and a conjugated system forms with ability to absorb photons of visible light, and therefore appear colorful. When the UV source is removed, the molecules gradually relax to their ground state, the carbon-oxygen bond
Carbon-oxygen bond
A carbon–oxygen bond is a covalent bond between carbon and oxygen and one of the most abundant in organic chemistry and biochemistry. Oxygen has 6 valence electrons and prefers to share two electrons in bonding with carbon, leaving the remaining 4 nonbonding electrons in 2 lone pairs...
reforms, the spiro-carbon becomes sp³ hybridized again, and the molecule returns to its colorless state.
This class of photochromes in particular are thermodynamically unstable in one form and revert to the stable form in the dark unless cooled to low temperatures. Their lifetime can also be affected by exposure to UV light. Like most organic dye
Dye
A dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
s they are susceptible to degradation by oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and free radicals. Incorporation of the dyes into a polymer matrix, adding a stabilizer, or providing a barrier to oxygen and chemicals by other means prolongs their lifetime.
Diarylethenes
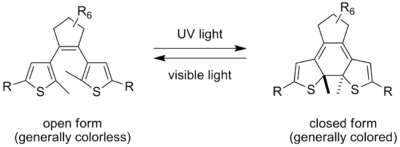
Diarylethene
In chemistry, diarylethene is the general name of a class of compounds that have aromatic groups bonded to each end of a carbon-carbon double bond...
s" were first introduced by Irie and have since gained widespread interest, largely on account of their high thermodynamic stability. They operate by means of a 6-pi electrocyclic reaction
Electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
, the thermal analog of which is impossible due to steric hindrance. Pure photochromic dyes usually have the appearance of a crystalline powder, and in order to achieve the color change, they usually have to be dissolved in a solvent or dispersed in a suitable matrix. However, some diarylethenes have so little shape change upon isomerization that they can be converted while remaining in crystalline form.
Azobenzenes
The photochromic trans-cis isomerization of azobenzeneAzobenzene
Azobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
s has been used extensively in molecular switch
Molecular switch
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to changes in e.g. pH, light, temperature, an electrical current, microenvironment, or the presence of a ligand. In some cases, a...
es, often taking advantage of its shape change upon isomerization to produce a supramolecular result. In particular, azobenzenes incorporated into crown ethers give switchable receptors and azobenzenes in monolayer
Monolayer
- Chemistry :A Langmuir monolayer or insoluble monolayer is a one-molecule thick layer of an insoluble organic material spread onto an aqueous subphase. Traditional compounds used to prepare Langmuir monolayers are amphiphilic materials that possess a hydrophilic headgroup and a hydrophobic tail...
s can provide light-controlled changes in surface properties.
Photochromic quinones
Some quinones, and phenoxynaphthacene quinone in particular, have photochromicity resulting from the ability of the phenyl group to migrate from one oxygen atome to another. Quinones with good thermal stability have been prepared, and they also have the additional feature of redox activity, leading to the construction of many-state molecular switches that operate by a mixture of photonic and electronic stimuli.Inorganic photochromics
Many inorganic substances also exhibit photochromic properties, often with much better resistance to fatigue than organic photochromics. In particular, silver chlorideSilver chloride
Silver chloride is a chemical compound with the chemical formula AgCl. This white crystalline solid is well known for its low solubility in water . Upon illumination or heating, silver chloride converts to silver , which is signalled by greyish or purplish coloration to some samples...
is extensively used in the manufacture of photochromic lenses
Photochromic lenses
Photochromic lenses are lenses that darken on exposure to ultraviolet radiation. Once the UV is removed , the lenses will gradually return to their clear state...
. Other silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
and zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
halides are also photochromic.
Sunglasses
One of the most famous reversible photochromic applications is color changing lenses for sunglassesSunglasses
Sunglasses or sun glasses are a form of protective eyewear designed primarily to prevent bright sunlight and high-energy visible light from damaging or discomforting the eyes. They can sometimes also function as a visual aid, as variously termed spectacles or glasses exist, featuring lenses that...
, as found in eye-glasses. The largest limitation in using PC technology is that the materials cannot be made stable enough to withstand thousands of hours of outdoor exposure so long-term outdoor applications are not appropriate at this time.
The switching speed of photochromic dyes is highly sensitive to the rigidity of the environment around the dye. As result, they switch most rapidly in solution and slowest in the rigid environment like a polymer lens. In 2005 it was reported that attaching flexible polymers with low glass transition temperature (for example siloxanes or poly(butyl acrylate)) to the dyes allow them to switch much more rapidly in a rigid lens. Some spirooxazines with siloxane polymers attached switch at near solution-like speeds even though they are in a rigid lens matrix.
Supramolecular chemistry
Photochromic units have been employed extensively in supramolecular chemistrySupramolecular chemistry
Supramolecular chemistry refers to the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components...
. Their ability to give a light-controlled reversible shape change means that they can be used to make or break molecular recognition motifs, or to cause a consequent shape change in their surroundings. Thus, photochromic units have been demonstrated as components of molecular switch
Molecular switch
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to changes in e.g. pH, light, temperature, an electrical current, microenvironment, or the presence of a ligand. In some cases, a...
es. The coupling of photochromic units to enzymes or enzyme cofactors even provides the ability to reversibly turn enzymes "on" and "off", by altering their shape or orientation in such a way that their functions are either "working" or "broken".
Data storage
The possibility of using photochromic compounds for data storageData storage device
thumb|200px|right|A reel-to-reel tape recorder .The magnetic tape is a data storage medium. The recorder is data storage equipment using a portable medium to store the data....
was first suggested in 1956 by Yehuda Hirshberg. Since that time, there have been many investigations by various academic and commercial groups, particularly in the area of 3D optical data storage
3D optical data storage
3D optical data storage is the term given to any form of optical data storage in which information can be recorded and/or read with three dimensional resolution ....
which promises discs that can hold a terabyte
Terabyte
The terabyte is a multiple of the unit byte for digital information. The prefix tera means 1012 in the International System of Units , and therefore 1 terabyte is , or 1 trillion bytes, or 1000 gigabytes. 1 terabyte in binary prefixes is 0.9095 tebibytes, or 931.32 gibibytes...
of data. Initially, issues with thermal back-reaction
Back-reaction
In theoretical physics, Back-reaction is often necessary to calculate the behavior of a particle or an object in an external field.When the particle is considered to be infinitely light or have an infinitesimal charge, it is said that we deal with a probe and the back-reaction is neglected...
s and destructive reading dogged these studies, but more recently more-stable systems have been developed.
Novelty items
Reversible photochromics are also found in applications such as toyToy
A toy is any object that can be used for play. Toys are associated commonly with children and pets. Playing with toys is often thought to be an enjoyable means of training the young for life in human society. Different materials are used to make toys enjoyable and cuddly to both young and old...
s, cosmetics
Cosmetics
Cosmetics are substances used to enhance the appearance or odor of the human body. Cosmetics include skin-care creams, lotions, powders, perfumes, lipsticks, fingernail and toe nail polish, eye and facial makeup, towelettes, permanent waves, colored contact lenses, hair colors, hair sprays and...
, clothing
Clothing
Clothing refers to any covering for the human body that is worn. The wearing of clothing is exclusively a human characteristic and is a feature of nearly all human societies...
and industrial applications. If necessary, they can be made to change between desired colors by combination with a permanent pigment
Pigment
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
.

