Carbon-oxygen bond
Encyclopedia
A carbon–oxygen bond is a covalent bond
between carbon
and oxygen
and one of the most abundant in organic chemistry
and biochemistry
. Oxygen has 6 valence electron
s and prefers to share two electrons in bonding with carbon, leaving the remaining 4 nonbonding electrons in 2 lone pair
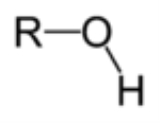
s. The simplest representatives can be thought of organic derivatives of water
: the alcohol
s.
A C–O bond is strongly polarized towards oxygen (electronegativity
C vs O = 2.55:3.44). Bond length
s for paraffinic C–O bonds are in the range of 143 picometer – less than those
of C–N or C–C bonds. Shortened single bonds are found with carboxylic acids (136 pm) due to partial double bond character and elongated bonds are found in epoxide
s (147 pm). The C–O bond strength
is also larger than C–N or C–C. For example, bond strengths are 91 kcal/mol (at 298 K) in methanol
, 87 kcal/mol in methylamine
, and 88 kcal/mol in ethane
.
Carbon and oxygen form terminal double bond
s in functional groups collectively known as carbonyl
compounds to which belong such compounds as ketone
s, ester
s, carboxylic acid
s and many more. Internal C=O bonds are found in positively charged oxonium ion
s, but occur mainly as reactive intermediate
s. In furan
s, the oxygen atom contributes to pi-electron delocalization via its filled p-orbital and hence furans are aromatic
. Bond lengths of C=O bonds are around 123 pm in carbonyl compounds. The C=O bonds in acyl halide
s have partial triple bond
character and subsequently very short: 117 pm. Compounds with formal C–O triple bonds do not exist except for carbon monoxide
, which has a very short, strong bond (112.8 pm). Such triple bonds have a very high bond energy, even higher than N–N triple bonds. Oxygen can also be trivalent , for example in triethyloxonium tetrafluoroborate.
, nucleophilic acyl substitution
s and electrophilic addition
to alkenes. The Paternò–Büchi reaction is the carbonyl equivalent of a metathesis reaction.
s:
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
between carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and one of the most abundant in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
and biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
. Oxygen has 6 valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s and prefers to share two electrons in bonding with carbon, leaving the remaining 4 nonbonding electrons in 2 lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
s. The simplest representatives can be thought of organic derivatives of water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
: the alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s.
A C–O bond is strongly polarized towards oxygen (electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
C vs O = 2.55:3.44). Bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s for paraffinic C–O bonds are in the range of 143 picometer – less than those
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
of C–N or C–C bonds. Shortened single bonds are found with carboxylic acids (136 pm) due to partial double bond character and elongated bonds are found in epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s (147 pm). The C–O bond strength
Bond strength
In chemistry, bond strength is measured between two atoms joined in a chemical bond. It is the degree to which each atom linked to another atom contributes to the valency of this other atom...
is also larger than C–N or C–C. For example, bond strengths are 91 kcal/mol (at 298 K) in methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, 87 kcal/mol in methylamine
Methylamine
Methylamine is the organic compound with a formula of CH3NH2. This colourless gas is a derivative of ammonia, but with one H atom replaced by a methyl group. It is the simplest primary amine. It is sold as a solution in methanol, ethanol, THF, and water, or as the anhydrous gas in pressurized...
, and 88 kcal/mol in ethane
Ethane
Ethane is a chemical compound with chemical formula C2H6. It is the only two-carbon alkane that is an aliphatic hydrocarbon. At standard temperature and pressure, ethane is a colorless, odorless gas....
.
Carbon and oxygen form terminal double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s in functional groups collectively known as carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
compounds to which belong such compounds as ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s, ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s, carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s and many more. Internal C=O bonds are found in positively charged oxonium ion
Oxonium ion
The oxonium ion in chemistry is any oxygen cation with three bonds. The simplest oxonium ion is the hydronium ion H3O+. Another oxonium ion frequently encountered in organic chemistry is obtained by protonation or alkylation of a carbonyl group e.g...
s, but occur mainly as reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
s. In furan
Furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans....
s, the oxygen atom contributes to pi-electron delocalization via its filled p-orbital and hence furans are aromatic
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
. Bond lengths of C=O bonds are around 123 pm in carbonyl compounds. The C=O bonds in acyl halide
Acyl halide
An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group....
s have partial triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
character and subsequently very short: 117 pm. Compounds with formal C–O triple bonds do not exist except for carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, which has a very short, strong bond (112.8 pm). Such triple bonds have a very high bond energy, even higher than N–N triple bonds. Oxygen can also be trivalent , for example in triethyloxonium tetrafluoroborate.
Chemistry
Important carbon–oxygen bond forming reactions are the Williamson ether synthesisWilliamson ether synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and an alcohol. This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction...
, nucleophilic acyl substitution
Nucleophilic acyl substitution
Nucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
s and electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
to alkenes. The Paternò–Büchi reaction is the carbonyl equivalent of a metathesis reaction.
Oxygen functional groups
Carbon–oxygen bonds are present in these functional groupFunctional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s:
| Chemical class | Bond order Bond order Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond.... |
Formula | Structural Formula | Example |
|---|---|---|---|---|
| Alcohol Alcohol In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms.... s |
1 | R3C–OH | -skeletal.png) |
 Ethanol Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a... |
| Ether Ether Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"... s |
1 | R3C–O–CR3 | .png) |
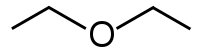 Diethyl ether Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor... |
| Peroxide Peroxide A peroxide is a compound containing an oxygen–oxygen single bond or the peroxide anion .The O−O group is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.The simplest stable peroxide is hydrogen peroxide... s |
1 | R3C–O–O–CR3 |  |
 |
| Ester Ester Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and... s |
1 | R3C–CO–O–CR3 |  |
 Ethyl acrylate Ethyl acrylate is an organic compound primarily used in the preparation of various polymers. It is a clear liquid with an acrid penetrating odor. The human nose is capable of detecting this odor at a thousand times lower concentration than is considered harmful if continuously exposed for some... |
| Carbonate ester Carbonate ester A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as... s |
1 | R3C–O–CO–O–CR3 |  |
 Ethylene carbonate Ethylene carbonate is an ester of ethylene glycol and carbonic acid. At room temperature ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water. In the liquid state Ethylene carbonate is an ester of ethylene glycol and carbonic... |
| Ketone Ketone In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology... s |
2 | R3C–CO–CR3 | 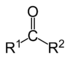 |
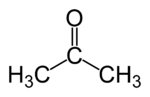 Acetone Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory... |
| Aldehyde Aldehyde An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group.... s |
2 | R3C–CHO | 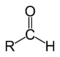 |
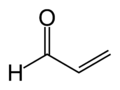 Acrolein Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity... |
| Furan Furan Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen. The class of compounds containing such rings are also referred to as furans.... s |
1.5 | 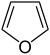 |
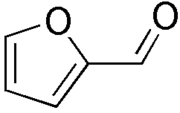 Furfural Furfural is an organic compound derived from a variety of agricultural byproducts, including corncobs, oat, wheat bran, and sawdust. The name furfural comes from the Latin word , meaning bran, referring to its usual source.... |
|
| Pyrylium salt Pyrylium salt A pyrylium salt is a salt containing a pyrylium cation or a derivative of it. The pyrylium cation is a conjugated 6-membered carbon ring system with one carbon atom replaced by a positively charged oxygen atom. It is, like benzene, an aromatic compound... s |
1.5 |  |
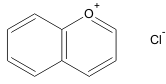 Anthocyanin Anthocyanins are water-soluble vacuolar pigments that may appear red, purple, or blue according to pH... s |
|
See also
- The chemistry of carbon bonded to other elements in the periodic table:

