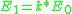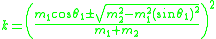Rutherford backscattering spectrometry
Encyclopedia
Rutherford backscattering spectrometry (RBS) is an analytical technique used in materials science
. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions impinging on a sample.
 Rutherford backscattering spectrometry is named after Lord Ernest Rutherford
Rutherford backscattering spectrometry is named after Lord Ernest Rutherford
, a physicist
sometimes referred to as the father of nuclear physics
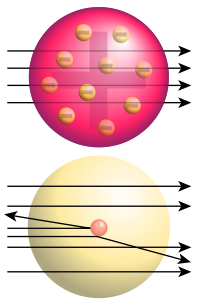
. Rutherford supervised a series of experiments carried out by Hans Geiger and Ernest Marsden
between 1909 and 1914 studying the scattering of alpha particles through metal foils. While attempting to eliminate "stray particles" they believed to be caused by an imperfection in their alpha source, Rutherford suggested that Marsden attempt to measure backscattering from a gold foil sample. According to the then-dominant plum-pudding model of the atom, in which small negative electrons were spread through a diffuse positive region, backscattering of the high-energy positive alpha particles should have been nonexistent. At most small deflections should occur as the alpha particles passed almost unhindered through the foil. Instead, when Marsden positioned the detector on the same side of the foil as the alpha particle source, he immediately detected a noticeable backscattered signal. According to Rutherford, "It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it
came back and hit you."
Rutherford interpreted the result of the Geiger-Marsden experiment
as an indication of a Coulomb collision
with a single massive positive particle. This led him to the conclusion that the atom's positive charge could not be diffuse but instead must be concentrated in a single massive core: the atomic nucleus
. Calculations indicated that the charge necessary to accomplish this deflection was approximately 100 times the charge of the electron, close to the atomic number of gold. This led to the development of the Rutherford model
of the atom in which a positive nucleus made up of Ne positive particles, or protons, was surrounded by N orbiting electrons of charge e to balance the nuclear charge. This model was eventually superseded by the Bohr atom, incorporating some early results from quantum mechanics
.
If the energy of the incident particle is increased sufficiently, the Coulomb barrier
is exceeded and the wavefunctions of the incident and struck particles overlap. This may result in nuclear reactions
in certain cases, but frequently the interaction remains elastic
, although the scattering cross-sections may fluctuate wildly as a function of energy. This case is known as "Elastic (non-Rutherford) Backscattering Spectrometry" (EBS). There has recently been enormous progress in determining EBS scattering cross-sections, by solving Schrödinger's equation
for each interaction (see http://www-nds.iaea.org/sigmacalc/).
(hard-sphere
) collision between a high kinetic energy particle from the incident beam (the projectile) and a stationary particle located in the sample (the target). Elastic in this context means that no energy is either lost or gained during the collision.
Note that the "law" of the conservation of energy
is not generally applicable for nuclear interactions, since in some circumstances a collision may result in a nuclear reaction, with the release of what can be very considerable quantities of energy. Nuclear reaction analysis
(NRA) is very useful for detecting light elements. Of course, even for NRA the conservation law still applies, but in the more general mass-energy form.
Considering the kinematics
of the collision (that is, the conservation of momentum and kinetic energy), the energy of the scattered projectile is reduced from the initial energy
of the scattered projectile is reduced from the initial energy 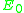 :
:
where k is known as the kinematical factor, and
where particle 1 is the projectile, particle 2 is the target nucleus, and is the scattering angle of the projectile in the laboratory frame of reference
is the scattering angle of the projectile in the laboratory frame of reference
(that is, relative to the observer). The plus sign is taken when the mass of the projectile is less than that of the target, otherwise the minus sign is taken.
While this equation correctly determines the energy of the scattered projectile for any particular scattering angle (relative to the observer), it does not describe the probability of observing such an event. For that we need the differential cross-section of the backscattering event:
Materials science
Materials science is an interdisciplinary field applying the properties of matter to various areas of science and engineering. This scientific field investigates the relationship between the structure of materials at atomic or molecular scales and their macroscopic properties. It incorporates...
. Sometimes referred to as high-energy ion scattering (HEIS) spectrometry, RBS is used to determine the structure and composition of materials by measuring the backscattering of a beam of high energy ions impinging on a sample.
The Geiger–Marsden experiment

Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics...
, a physicist
Physicist
A physicist is a scientist who studies or practices physics. Physicists study a wide range of physical phenomena in many branches of physics spanning all length scales: from sub-atomic particles of which all ordinary matter is made to the behavior of the material Universe as a whole...
sometimes referred to as the father of nuclear physics
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
. Rutherford supervised a series of experiments carried out by Hans Geiger and Ernest Marsden
Ernest Marsden
Sir Ernest Marsden was an English-New Zealand physicist. He was born in East Lancashire, living in Rishton and educated at Queen Elizabeth's Grammar School, Blackburn, where an inter-house trophy rewarding academic excellence bears his name.He met Ernest Rutherford at the University of Manchester...
between 1909 and 1914 studying the scattering of alpha particles through metal foils. While attempting to eliminate "stray particles" they believed to be caused by an imperfection in their alpha source, Rutherford suggested that Marsden attempt to measure backscattering from a gold foil sample. According to the then-dominant plum-pudding model of the atom, in which small negative electrons were spread through a diffuse positive region, backscattering of the high-energy positive alpha particles should have been nonexistent. At most small deflections should occur as the alpha particles passed almost unhindered through the foil. Instead, when Marsden positioned the detector on the same side of the foil as the alpha particle source, he immediately detected a noticeable backscattered signal. According to Rutherford, "It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it
came back and hit you."
Rutherford interpreted the result of the Geiger-Marsden experiment
Geiger-Marsden experiment
The Geiger–Marsden experiment was an experiment to probe the structure of the atom performed by Hans Geiger and Ernest Marsden in 1909, under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester...
as an indication of a Coulomb collision
Coulomb collision
A Coulomb collision is a binary elastic collision between two charged particles interacting through their own Electric Field. As with any inverse-square law, the resulting trajectories of the colliding particles is a hyperbolic Keplerian orbit...
with a single massive positive particle. This led him to the conclusion that the atom's positive charge could not be diffuse but instead must be concentrated in a single massive core: the atomic nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
. Calculations indicated that the charge necessary to accomplish this deflection was approximately 100 times the charge of the electron, close to the atomic number of gold. This led to the development of the Rutherford model
Rutherford model
The Rutherford model or planetary model is a model of the atom devised by Ernest Rutherford. Rutherford directed the famous Geiger-Marsden experiment in 1909, which suggested on Rutherford's 1911 analysis that the so-called "plum pudding model" of J. J. Thomson of the atom was incorrect...
of the atom in which a positive nucleus made up of Ne positive particles, or protons, was surrounded by N orbiting electrons of charge e to balance the nuclear charge. This model was eventually superseded by the Bohr atom, incorporating some early results from quantum mechanics
Quantum mechanics
Quantum mechanics, also known as quantum physics or quantum theory, is a branch of physics providing a mathematical description of much of the dual particle-like and wave-like behavior and interactions of energy and matter. It departs from classical mechanics primarily at the atomic and subatomic...
.
If the energy of the incident particle is increased sufficiently, the Coulomb barrier
Coulomb barrier
The Coulomb barrier, named after Coulomb's law, which is named after physicist Charles-Augustin de Coulomb , is the energy barrier due to electrostatic interaction that two nuclei need to overcome so they can get close enough to undergo a nuclear reaction...
is exceeded and the wavefunctions of the incident and struck particles overlap. This may result in nuclear reactions
Nuclear reaction analysis
Nuclear reaction analysis is a nuclear method in materials science to obtain concentration vs. depth distributions for certain target chemical elements in a solid thin film....
in certain cases, but frequently the interaction remains elastic
Elastic collision
An elastic collision is an encounter between two bodies in which the total kinetic energy of the two bodies after the encounter is equal to their total kinetic energy before the encounter...
, although the scattering cross-sections may fluctuate wildly as a function of energy. This case is known as "Elastic (non-Rutherford) Backscattering Spectrometry" (EBS). There has recently been enormous progress in determining EBS scattering cross-sections, by solving Schrödinger's equation
Schrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
for each interaction (see http://www-nds.iaea.org/sigmacalc/).
Basic principles
We can describe Rutherford backscattering as an elasticElastic collision
An elastic collision is an encounter between two bodies in which the total kinetic energy of the two bodies after the encounter is equal to their total kinetic energy before the encounter...
(hard-sphere
Hard spheres
Hard spheres are widely used as model particles in the statistical mechanical theory of fluids and solids. They are defined simply as impenetrable spheres that cannot overlap in space. They mimic the extremely strong repulsion that atoms and spherical molecules experience at very close distances...
) collision between a high kinetic energy particle from the incident beam (the projectile) and a stationary particle located in the sample (the target). Elastic in this context means that no energy is either lost or gained during the collision.
Note that the "law" of the conservation of energy
Conservation of energy
The nineteenth century law of conservation of energy is a law of physics. It states that the total amount of energy in an isolated system remains constant over time. The total energy is said to be conserved over time...
is not generally applicable for nuclear interactions, since in some circumstances a collision may result in a nuclear reaction, with the release of what can be very considerable quantities of energy. Nuclear reaction analysis
Nuclear reaction analysis
Nuclear reaction analysis is a nuclear method in materials science to obtain concentration vs. depth distributions for certain target chemical elements in a solid thin film....
(NRA) is very useful for detecting light elements. Of course, even for NRA the conservation law still applies, but in the more general mass-energy form.
Considering the kinematics
Kinematics
Kinematics is the branch of classical mechanics that describes the motion of bodies and systems without consideration of the forces that cause the motion....
of the collision (that is, the conservation of momentum and kinetic energy), the energy
 of the scattered projectile is reduced from the initial energy
of the scattered projectile is reduced from the initial energy 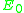 :
:where k is known as the kinematical factor, and
where particle 1 is the projectile, particle 2 is the target nucleus, and
 is the scattering angle of the projectile in the laboratory frame of reference
is the scattering angle of the projectile in the laboratory frame of referenceFrame of reference
A frame of reference in physics, may refer to a coordinate system or set of axes within which to measure the position, orientation, and other properties of objects in it, or it may refer to an observational reference frame tied to the state of motion of an observer.It may also refer to both an...
(that is, relative to the observer). The plus sign is taken when the mass of the projectile is less than that of the target, otherwise the minus sign is taken.
While this equation correctly determines the energy of the scattered projectile for any particular scattering angle (relative to the observer), it does not describe the probability of observing such an event. For that we need the differential cross-section of the backscattering event:
-

where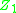 and
and 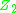 are the atomic numbers of the incident and target nuclei. This equation is written in the centre of massCenter of massIn physics, the center of mass or barycenter of a system is the average location of all of its mass. In the case of a rigid body, the position of the center of mass is fixed in relation to the body...
are the atomic numbers of the incident and target nuclei. This equation is written in the centre of massCenter of massIn physics, the center of mass or barycenter of a system is the average location of all of its mass. In the case of a rigid body, the position of the center of mass is fixed in relation to the body...
frame of referenceFrame of referenceA frame of reference in physics, may refer to a coordinate system or set of axes within which to measure the position, orientation, and other properties of objects in it, or it may refer to an observational reference frame tied to the state of motion of an observer.It may also refer to both an...
and is therefore not a function of the mass of either the projectile or the target nucleus.
Note that the scattering angle in the laboratory frame of reference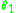 is not the same as the scattering angle in the centre of mass frame of reference
is not the same as the scattering angle in the centre of mass frame of reference  (although for RBS experiments they are usually very similar). However, heavy ion projectiles can easily recoilAtomic RecoilAtomic recoil is the result of the interaction of an atom with an energetic elementary particle, when the momentum of the interacting particle is transferred to the atom as whole without altering non-translational degrees of freedom of the atom...
(although for RBS experiments they are usually very similar). However, heavy ion projectiles can easily recoilAtomic RecoilAtomic recoil is the result of the interaction of an atom with an energetic elementary particle, when the momentum of the interacting particle is transferred to the atom as whole without altering non-translational degrees of freedom of the atom...
lighter ions which, if the geometry is right, can be ejected from the target and detected. This is the basis of the Elastic Recoil Detection (ERD, with synonyms ERDA, FRS, HFS) technique. RBS often uses a He beam which readily recoils H, so simultaneous RBS/ERD is frequently done to probe the hydrogen isotope content of samples (although H ERD with a He beam above 1 MeV is not Rutherford: see http://www-nds.iaea.org/sigmacalc). For ERD the scattering angle in the lab frame of reference is quite different from that in the centre of mass frame of reference.
Note also that heavy ions cannot backscatter from light ones: it is kinematically prohibited. The kinematical factor must remain real, and this limits the permitted scattering angle in the laboratory frame of reference. In ERD it is often convenient to place the recoil detector at recoil angles large enough to prohibit signal from the scattered beam. The scattered ion intensity is always very large compared to the recoil intensity (the Rutherford scattering cross-section formula goes to infinity as the scattering angle goes to zero), and for ERD the scattered beam usually has to be excluded from the measurement somehow.
The singularity in the Rutherford scattering cross-section formula is unphysical of course. If the scattering cross-section is zero it implies that the projectile never comes close to the target, but in this case it also never penetrates the electron cloud surrounding the nucleus either. The pure Coulomb formula for the scattering cross-section shown above must be corrected for this screening effectShielding effectThe shielding effect describes the decrease in attraction between an electron and the nucleus in any atom with more than one electron shell. It is also referred to as the screening effect or atomic shielding.-Cause:...
, which becomes more important as the energy of the projectile decreases (or, equivalently, its mass increases).
While large-angle scattering only occurs for ions which scatter off target nuclei, inelastic small-angle scattering can also occur off the sample electrons. This results in a gradual decrease in ions which penetrate more deeply into the sample, so that backscattering off interior nuclei occurs with a lower "effective" incident energy. The amount by which the ion energy is lowered after passing through a given distance is referred to as the stopping powerStopping power (particle radiation)In passing through matter, fast charged particles ionize the atoms or molecules which they encounter. Thus, the fast particles gradually lose energy in many small steps. Stopping power is defined as the average energy loss of the particle per unit path length, measured for example in MeV/cm...
of the material and is dependent on the electron distribution. This energy loss varies continuously with respect to distance traversed, so that stopping power is expressed as
For high energy ions stopping power is usually proportional to ; however, precise calculation of stopping power is difficult to carry out with any accuracy.
; however, precise calculation of stopping power is difficult to carry out with any accuracy.
Stopping power (properly, stopping force) has units of energy per unit length. It is generally given in thin film units, that is eV /(atom/cm2) since it is measured experimentally on thin films whose thickness is always measured absolutely as mass per unit area, avoiding the problem of determining the density of the material which may vary as a function of thickness. Stopping power is now known for all materials at around 2%, see http://www.srim.org.
Instrumentation
An RBS instrument generally includes three essential components:
- An ionIonAn ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
source, usually alpha particleAlpha particleAlpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
s (He2+ ions) or, less commonly, protonProtonThe proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s. - A linear particle acceleratorLinear particle acceleratorA linear particle accelerator is a type of particle accelerator that greatly increases the velocity of charged subatomic particles or ions by subjecting the charged particles to a series of oscillating electric potentials along a linear beamline; this method of particle acceleration was invented...
capable of accelerating incident ions to high energies, usually in the range 1-3 MeV. - A detector capable of measuring the energies of backscatterBackscatterIn physics, backscatter is the reflection of waves, particles, or signals back to the direction they came from. It is a diffuse reflection due to scattering, as opposed to specular reflection like a mirror...
ed ions over some range of angles.
Two common source/acceleration arrangements are used in commercial RBS systems, working in either one or two stages. One-stage systems consist of a He+ source connected to an acceleration tube with a high positive potential applied to the ion source, and the ground at the end of the acceleration tube. This arrangement is simple and convenient, but it can be difficult to achieve energies of much more than 1 MeV due to the difficulty of applying very high voltages to the system.
Two-stage systems, or "tandem accelerators", start with a source of He- ions and position the positive terminal at the center of the acceleration tube. A stripper element included in the positive terminal removes electrons from ions which pass through, converting He- ions to He++ ions. The ions thus start out being attracted to the terminal, pass through and become positive, and are repelled until they exit the tube at ground. This arrangement, though more complex, has the advantage of achieving higher accelerations with lower applied voltages: a typical tandem accelerator with an applied voltage of 750 kV can achieve ion energies of over 2 MeV.
Detectors to measure backscattered energy are usually siliconSiliconSilicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
surface barrier detectors, a very thin layer (100 nm) of P-typeP-type semiconductorA P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
silicon on an N-typeN-type semiconductorN-type semiconductors are a type of extrinsic semiconductor where the dopant atoms are capable of providing extra conduction electrons to the host material . This creates an excess of negative electron charge carriers....
substrate forming a p-n junctionP-n junctionA p–n junction is formed at the boundary between a P-type and N-type semiconductor created in a single crystal of semiconductor by doping, for example by ion implantation, diffusion of dopants, or by epitaxy .If two separate pieces of material were used, this would...
. Ions which reach the detector lose some of their energy to inelastic scatteringInelastic scatteringIn particle physics and chemistry, inelastic scattering is a fundamental scattering process in which the kinetic energy of an incident particle is not conserved . In an inelastic scattering process, some of the energy of the incident particle is lost or gained...
from the electrons, and some of these electrons gain enough energy to overcome the band gapBand gapIn solid state physics, a band gap, also called an energy gap or bandgap, is an energy range in a solid where no electron states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference between the top of the valence band and the...
between the semiconductor valenceValence bandIn solids, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature....
and conduction bandConduction bandIn the solid-state physics field of semiconductors and insulators, the conduction band is the range of electron energies, higher than that of the valence band, sufficient to free an electron from binding with its individual atom and allow it to move freely within the atomic lattice of the material...
s. This means that each ion incident on the detector will produce some number of electron-hole pairsCarrier generation and recombinationIn the solid state physics of semiconductors, carrier generation and recombination are processes by which mobile charge carriers are created and eliminated. Carrier generation and recombination processes are fundamental to the operation of many optoelectronic semiconductor devices, such as...
which is dependent on the energy of the ion. These pairs can be detected by applying a voltage across the detector and measuring the current, providing an effective measurement of the ion energy. The relationship between ion energy and the number of electron-hole pairs produced will be dependent on the detector materials, the type of ion and the efficiency of the current measurement; energy resolution is dependent on thermal fluctuations. It should also be noted that after one ion is incident on the detector, there will be some dead timeDead timeFor detection systems that record discrete events, such as particle and nuclear detectors, the dead time is the time after each event during which the system is not able to record another event....
before the electron-hole pairs recombine in which a second incident ion cannot be distinguished from the first.
Angular dependence of detection can be achieved by using a movable detector, or more practically by separating the surface barrier detector into many independent cells which can be measured independently, covering some range of angles around direct (180 degrees) backscattering. Angular dependence of the incident beam is controlled by using a tiltable sample stage.
Composition and depth measurement
The energy loss of a backscattered ion is dependent on two processes: the energy lost in scattering events with sample nuclei, and the energy lost to small-angle scattering from the sample electrons. The first process is dependent on the scattering cross-section of the nucleus and thus on its mass and atomic number. For a given measurement angle, nuclei of two different elements will therefore scatter incident ions to different degrees and with different energies, producing separate peaks on an N(E) plot of measurement count versus energy. These peaks are characteristic of the elements contained in the material, providing a means of analyzing the composition of a sample by matching scattered energies to known scattering cross-sections. Relative concentrations can be determined by measuring the heights of the peaks.
The second energy loss process, the stopping power of the sample electrons, does not result in large discrete losses such as those produced by nuclear collisions. Instead it creates a gradual energy loss dependent on the electron density and the distance traversed in the sample. This energy loss will lower the measured energy of ions which backscatter from nuclei inside the sample in a continuous manner dependent on the depth of the nuclei. The result is that instead of the sharp backscattered peaks one would expect on an N(E) plot, with the width determined by energy and angular resolution, the peaks observed trail off gradually towards lower energy as the ions pass through the depth occupied by that element. Elements which only appear at some depth inside the sample will also have their peak positions shifted by some amount which represents the distance an ion had to traverse to reach those nuclei.
In practice, then, a compositional depth profile can be determined from an RBS N(E) measurement. The elements contained by a sample can be determined from the positions of peaks in the energy spectrum. Depth can be determined from the width and shifted position of these peaks, and relative concentration from the peak heights. This is especially useful for the analysis of a multilayer sample, for example, or for a sample with a composition which varies more continuously with depth.
This kind of measurement can only be used to determine elemental composition; the chemical structure of the sample cannot be determined from the N(E) profile. However, it is possible to learn something about this through RBS by examining the crystal structure. This kind of spatial information can be investigated by taking advantage of blocking and channeling.
Structural measurements: blocking and channeling
To fully understand the interaction of an incident beam of nuclei with a crystalline structure, we need two more key concepts: blocking and channelingChannelingChanneling, or channelling, can refer toscience*Channelling , the process that constrains the path of a charged particle in a crystalline solid.*metabolite or substrate channeling in biochemistry and cell physiology.law...
.
When a beam of ions with parallel trajectories is incident on a target atom, scattering off that atom will prevent collisions in a cone-shaped region "behind" the target relative to the beam. This occurs because the repulsive potential of the target atom bends close ion trajectories away from their original path, and is referred to as blocking. The radius of this blocked region, at a distance L from the original atom, is given by
When an ion is scattered from deep inside a sample it can then re-scatter off a second atom, creating a second blocked cone in the direction of the scattered trajectory. This can be detected by carefully varying the detection angle relative to the incident angle.
ChannelingChannelingChanneling, or channelling, can refer toscience*Channelling , the process that constrains the path of a charged particle in a crystalline solid.*metabolite or substrate channeling in biochemistry and cell physiology.law...
is observed when the incident beam is aligned with a major symmetry axis of the crystal. Incident nuclei which avoid collisions with surface atoms are excluded from collisions with all atoms deeper in the sample, due to blocking by the first layer of atoms. When the interatomic distance is large compared to the radius of the blocked cone, the incident ions can penetrate many times the interatomic distance without being backscattered. This can result in a drastic reduction of the observed backscattered signal when the incident beam is oriented along a one of the symmetry directions, allowing determination of a sample's regular crystal structure. Channeling works best for very small blocking radii, i.e. for high-energy low-atomic-number incident ions such as He+.
The tolerance for the deviation of the ion beam angle of incidence relative to the symmetry direction depends on the blocking radius, making the allowable deviation angle proportional to
While the intensity of an RBS peak is observed to decrease across most of its width when the beam is channeled, a narrow peak at the high-energy end of larger peak will often be observed, representing surface scattering from the first layer of atoms. The presence of this peak opens the possibility of surface sensitivity for RBS measurements.
Profiling of Displaced Atoms
In addition, channeling of ions can also be used to analyze a crystalline sample for lattice damage . If atoms within the target are displaced from their crystalline lattice site this will result in a higher backscattering yield in relation to a perfect crystal. By comparing the spectrum from a sample being analyzed to that from a perfect crystal, and that obtained at a random (non-channeling) orientation (representative of a spectrum from an amorphous sample), it is possible to determine the extent of crystalline damage in terms of a fraction of displaced atoms. Multiplying this fraction by the density of the material when amorphous then also gives an estimate for the concentration of displaced atoms. The energy at which the increased backscattering occurs at can also be used to determine the depth at which the displaced atoms are and a defect depth profile can be built up as a result.
Surface sensitivity
While RBS is generally used to measure the bulk composition and structure of a sample, it is possible to obtain some information about the structure and composition of the sample surface. When the signal is channeled to remove the bulk signal, careful manipulation of the incident and detection angles can be used to determine the relative positions of the first few layers of atoms, taking advantage of blocking effects.
The surface structure of a sample can be changed from the ideal in a number of ways. The first layer of atoms can change its distance from subsequent layers (relaxation); it can assume a different two-dimensional structure than the bulk (reconstructionSurface reconstructionSurface reconstruction refers to the process by which atoms at the surface of a crystal assume a different structure than that of the bulk. Surface reconstructions are important in that they help in the understanding of surface chemistry for various materials, especially in the case where another...
); or another material can be adsorbedAdsorptionAdsorption is the adhesion of atoms, ions, biomolecules or molecules of gas, liquid, or dissolved solids to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. It differs from absorption, in which a fluid permeates or is dissolved by a liquid or solid...
onto the surface. Each of these cases can be detected by RBS. For example, surface reconstruction can be detected by aligning the beam in such a way that channeling should occur, so that only a surface peak of known intensity should be detected. A higher-than-usual intensity or a wider peak will indicate that the first layers of atoms are failing to block the layers beneath, i.e. that the surface has been reconstructed. Relaxations can be detected by a similar procedure with the sample tilted so the ion beam is incident at an angle selected so that first-layer atoms should block backscattering at a diagonal; that is, from atoms which are below and displaced from the blocking atom. A higher-than-expected backscattered yield will indicate that the first layer has been displaced relative to the second layer, or relaxed. Adsorbate materials will be detected by their different composition, changing the position of the surface peak relative to the expected position.
RBS has also been used to measure processes which affect the surface differently than the bulk by analyzing changes in the channeled surface peak. A well-known example of this is the RBS analysis of the premelting of lead surfaces by Frenken, Maree and van der Veen. In an RBS measurement of the Pb(110)Miller indexMiller indices form a notation system in crystallography for planes and directions in crystal lattices.In particular, a family of lattice planes is determined by three integers h, k, and ℓ, the Miller indices. They are written , and each index denotes a plane orthogonal to a direction in the...
surface, a well-defined surface peak which is stable at low temperatures was found to become wider and more intense as temperature increase past two-thirds of the bulk melting temperature. The peak reached the bulk height and width as temperature reached the melting temperature. This increase in the disorder of the surface, making deeper atoms visible to the incident beam, was interpreted as pre-melting of the surface, and computer simulations of the RBS process produced similar results when compared with theoretical pre-melting predictions.
RBS has also been combined with nuclear microscopyNuclear microscopyNuclear microscopy uses a device called a microprobe. A microprobe is a device that uses electromagnetic or electrostatic lenses to focus an ion beam. In this way a microprobe is very similar to a scanning electron microscope. One difference is that the nuclear microprobe's beam is usually composed...
, in which a focused ion beam is scanned across a surface in a manner similar to a scanning electron microscopeScanning electron microscopeA scanning electron microscope is a type of electron microscope that images a sample by scanning it with a high-energy beam of electrons in a raster scan pattern...
. The energetic analysis of backscattered signals in this kind of application provides compositional information about the surface, while the microprobe itself can be used to examine features such as periodic surface structures.
See also
- Rutherford scatteringRutherford scatteringIn physics, Rutherford scattering is a phenomenon that was explained by Ernest Rutherford in 1911, and led to the development of the Rutherford model of the atom, and eventually to the Bohr model. It is now exploited by the materials analytical technique Rutherford backscattering...
- Secondary ion mass spectrometrySecondary ion mass spectrometrySecondary ion mass spectrometry is a technique used in materials science and surface science to analyze the composition of solid surfaces and thin films by sputtering the surface of the specimen with a focused primary ion beam and collecting and analyzing ejected secondary ions...
- Ion beam analysisIon beam analysisIon beam analysis is an important family of modern analytical techniques involving the use of MeV ion beams to probe the composition and obtain elemental depth profiles in the near-surface layer of solids. All IBA methods are highly sensitive and allow the detection of elements in the...
- Nuclear reaction analysisNuclear reaction analysisNuclear reaction analysis is a nuclear method in materials science to obtain concentration vs. depth distributions for certain target chemical elements in a solid thin film....
- Elastic recoil detectionElastic recoil detectionElastic Recoil Detection, also referred to as forward recoil scattering, is a nuclear technique in materials science to obtain elemental concentration depth profiles in thin films. An energetic ion beam is directed at the sample to be depth profiled and there is an elastic nuclear interaction...
- Particle induced X-ray emission
- Nuclear microscopyNuclear microscopyNuclear microscopy uses a device called a microprobe. A microprobe is a device that uses electromagnetic or electrostatic lenses to focus an ion beam. In this way a microprobe is very similar to a scanning electron microscope. One difference is that the nuclear microprobe's beam is usually composed...
- Surface scienceSurface scienceSurface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid-gas interfaces. It includes the fields of surface chemistry and surface physics. Some related...
- Stopping power (particle radiation)Stopping power (particle radiation)In passing through matter, fast charged particles ionize the atoms or molecules which they encounter. Thus, the fast particles gradually lose energy in many small steps. Stopping power is defined as the average energy loss of the particle per unit path length, measured for example in MeV/cm...
- Collision cascadeCollision cascadeA collision cascade is a set of nearby adjacent energetic collisions of atoms induced by an energetic particle in a solid or liquid....
- Geiger–Marsden experiment
External links
- Rutherford Backscattering Spectrometry Theory Tutorial
- Rutherford Backscattering Instrumentation Tutorial
- RUMP - program for the simulation and analysis of RBS and ERD
- SIMNRA - program for the simulation and analysis of RBS, ERD and NRANRANRA is an abbreviation that may mean:* National regulatory authorities , government agencies tasked with regulating and supervising sections of public service and economy...
- DataFurnace - program for the simulation and analysis of RBS, ERD, NRANRANRA is an abbreviation that may mean:* National regulatory authorities , government agencies tasked with regulating and supervising sections of public service and economy...
, PIXEPIXEParticle-induced X-ray emission or proton-induced X-ray emission is a technique used in the determining of the elemental make-up of a material or sample. When a material is exposed to an ion beam, atomic interactions occur that give off EM radiation of wavelengths in the x-ray part of the...
and PIGE - NDF - free version of NDF (the calculation engine underlying DataFurnace) for the simulation of RBS, ERD and NRA
- IBANDL - ion beam analysis nuclear data library IAEA hosted and sponsored database of scattering cross-sections relevant to RBS and other IBAIBA-Iba:People*Clarence Iba , American basketball coach*Erol Iba , Indonesian footballer*Henry Iba , American basketball coach*Moe Iba , American basketball coach*Itsuki Iba, a fictional characterPlaces...
techniques - SigmaCalc IAEA hosted and sponsored site where evaluated elastic (non-Rutherford) scattering cross-sections can be calculated from Schrödinger's equationSchrödinger equationThe Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
for any scattering angle. - IBAsoft - Site including all simulations of the IAEA intercomparison of IBA software
- DEPTH - program to calculate the energy spread of detected particles from all depths in the target, and from causes including energy straggling, multiple scattering, and geometrical and kinematical broadening
- SRIM - the stopping and range of ions in matter - program to calculate stopping powers from tabulated parameters, based on a very large number of experimental measurements, evaluated (and interpolated) theoretically
- MSTAR - large collection of stopping powers, including a program to calculate heavy ion stopping
-