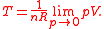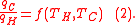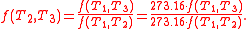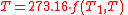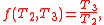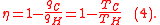Scale of temperature
Encyclopedia
Scale of temperature is a way to measure temperature quantitatively.
, being in thermal equilibrium is an equivalence relation
. Thus all thermal systems may be divided into a quotient set by this equivalence relation, denoted below as M. Assume the set M has the cardinality of c, then one can construct an injective function
ƒ: M → R , by which every thermal system will have a number associated with it such that when and only when two thermal systems have same such value, they will be in thermal equilibrium. This is clearly the property of temperature, and the specific way of assigning numerical values as temperature is called a scale of temperature.. In practical terms, a temperature scale is always based on usually a single physical property of a simple thermodynamic system, called a thermometer, that defines a scaling function mapping the temperature to the measurable thermometric parameter. Such temperature scales that are purely based on measurement are called empirical temperature scales.
The second law of thermodynamics
provides a fundamental, natural definition of thermodynamic temperature
starting with a null point of absolute zero
. A scale for thermodynamic temperature is established similarly to the empirical temperature scales, however, needing only one additional fixing point.
, provides the framework to measure temperature.
All temperature scales, including the modern thermodynamic temperature scale used in the International System of Units
, are calibrated according to thermal properties of a particular substance or device. Typically, this is established by fixing two well-defined temperature points and defining temperature increments via a linear function of the response of the thermometric device. For example, both the old Celsius scale and Fahrenheit scale were originally based on the linear expansion of a narrow mercury column within a limited range of temperature., each using different reference points and scale increments.
Different empirical scales may not be compatible with each other, except for small regions of temperature overlap. If an alcohol thermometer
and a mercury thermometer
have same two fixed points, namely the freezing and boiling point of water, their reading will not agree with each other except at the fixed points, as the linear 1:1 relationship of expansion between any two thermometric substances may not be guaranteed.
Empirical temperature scales are not reflective of the fundamental, microscopic laws of matter. Temperature is a universal attribute of matter, yet empirical scales map a narrow range onto a scale that is known to have a useful functional form for a particular application. Thus, their range is limited. The working material only exists in a form under certain circumstances, beyond which it no longer can serve as a scale. For example, mercury
freezes below 234.32 K, so temperature lower than that cannot be measured in a scale based on mercury. Even ITS-90, which interpolates among different ranges of temperature, has only a range of 0.65 K to approximately 1358 K (−272.5 °C to 1085 °C).
The ideal gas scale is in some sense a "mixed" scale. It relies on the universal properties of gas, a big advance from just a particular substance. But still it is empirical since it puts gas at a special position and thus has limited applicability—at some point no gas can exist. One distinguishing characteristic of ideal gas scale, however, is that it precisely equals thermodynamical scale when it is well defined (see below).
) as closely as possible throughout its range. Many different thermometer designs are required to cover the entire range. These include helium vapor pressure thermometers, helium gas thermometers, standard platinum resistance thermometers
(known as SPRTs, PRTs or Platium RTDs) and monochromatic radiation thermometers
.
Although the Kelvin and Celsius scales are defined using absolute zero (0 K) and the triple point
of water (273.16 K and 0.01 °C), it is impractical to use this definition at temperatures that are very different from the triple point of water. Accordingly, ITS–90 uses numerous defined points, all of which are based on various thermodynamic equilibrium states of fourteen pure chemical elements and one compound
(water). Most of the defined points are based on a phase transition
; specifically the melting
/freezing
point of a pure chemical element. However, the deepest cryogenic
points are based exclusively on the vapor pressure
/temperature relationship of helium and its isotopes whereas the remainder of its cold points (those less than room temperature) are based on triple point
s. Examples of other defining points are the triple point of hydrogen (−259.3467 °C) and the freezing point of aluminum (660.323 °C).
Thermometers calibrated per ITS–90 use complex mathematical formulas to interpolate between its defined points. ITS–90 specifies rigorous control over variables to ensure reproducibility from lab to lab. For instance, the small effect that atmospheric pressure has upon the various melting points is compensated for (an effect that typically amounts to no more than half a millikelvin across the different altitudes and barometric pressures likely to be encountered). The standard even compensates for the pressure effect due to how deeply the temperature probe is immersed into the sample. ITS–90 also draws a distinction between “freezing” and “melting” points. The distinction depends on whether heat is going into (melting) or out of (freezing) the sample when the measurement is made. Only gallium is measured while melting, all the other metals are measured while the samples are freezing.
There are often small differences between measurements calibrated per ITS–90 and thermodynamic temperature. For instance, precise measurements show that the boiling point of VSMOW
water under one standard atmosphere of pressure is actually 373.1339 K (99.9839 °C) when adhering strictly to the two-point definition of thermodynamic temperature. When calibrated to ITS–90, where one must interpolate between the defining points of gallium and indium, the boiling point of VSMOW water is about 10 mK less, about 99.974 °C. The virtue of ITS–90 is that another lab in another part of the world will measure the very same temperature with ease due to the advantages of a comprehensive international calibration standard featuring many conveniently spaced, reproducible, defining points spanning a wide range of temperatures.
scale that is named after the Swedish astronomer Anders Celsius
(1701–1744), who developed a similar temperature scale two years before his death. The degree Celsius (°C) can refer to a specific temperature on the Celsius scale as well as a unit to indicate a temperature interval
(a difference between two temperatures or an uncertainty
).
From 1744 until 1954, 0 °C was defined as the freezing point of water and 100 °C was defined as the boiling point of water, both at a pressure of one standard atmosphere
. Although these defining correlations are commonly taught in schools today, by international agreement the unit "degree Celsius" and the Celsius scale are currently defined by two different points: absolute zero
, and the triple point
of VSMOW (specially prepared water). This definition also precisely relates the Celsius scale to the Kelvin
scale, which defines the SI
base unit
of thermodynamic temperature
(symbol: K). Absolute zero, the hypothetical but unattainable temperature at which matter exhibits zero entropy, is defined as being precisely 0 K and −273.15 °C. The temperature value of the triple point of water is defined as being precisely 273.16 K and 0.01 °C.
This definition fixes the magnitude of both the degree Celsius and the kelvin as precisely 1 part in 273.16 parts, the difference between absolute zero and the triple point of water. Thus, it sets the magnitude of one degree Celsius and that of one kelvin as exactly the same. Additionally, it establishes the difference between the two scales' null points as being precisely 273.15 degrees Celsius (−273.15 °C = 0 K and 0 °C = 273.15 K).
). In experiments ITS-90 is used to approximate thermodynamic scale due to simpler realization.
The efficiency of a engine is the work divided by the heat introduced to the system or
where wcy is the work done per cycle. Thus, the efficiency depends only on qC/qH.
Because of Carnot theorem, any reversible heat engine operating between temperatures T1 and T2 must have the same efficiency, meaning, the efficiency is the function of the temperatures only:
In addition, a reversible heat engine operating between temperatures T1 and T3 must have the same efficiency as one consisting of two cycles, one between T1 and another (intermediate) temperature T2, and the second between T2andT3. This can only be the case if
Formal description
According to the zeroth law of thermodynamicsZeroth law of thermodynamics
The zeroth law of thermodynamics is a generalization principle of thermal equilibrium among bodies, or thermodynamic systems, in contact.The zeroth law states that if two systems are in thermal equilibrium with a third system, they are also in thermal equilibrium with each other.Systems are said to...
, being in thermal equilibrium is an equivalence relation
Equivalence relation
In mathematics, an equivalence relation is a relation that, loosely speaking, partitions a set so that every element of the set is a member of one and only one cell of the partition. Two elements of the set are considered equivalent if and only if they are elements of the same cell...
. Thus all thermal systems may be divided into a quotient set by this equivalence relation, denoted below as M. Assume the set M has the cardinality of c, then one can construct an injective function
Injective function
In mathematics, an injective function is a function that preserves distinctness: it never maps distinct elements of its domain to the same element of its codomain. In other words, every element of the function's codomain is mapped to by at most one element of its domain...
ƒ: M → R , by which every thermal system will have a number associated with it such that when and only when two thermal systems have same such value, they will be in thermal equilibrium. This is clearly the property of temperature, and the specific way of assigning numerical values as temperature is called a scale of temperature.. In practical terms, a temperature scale is always based on usually a single physical property of a simple thermodynamic system, called a thermometer, that defines a scaling function mapping the temperature to the measurable thermometric parameter. Such temperature scales that are purely based on measurement are called empirical temperature scales.
The second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
provides a fundamental, natural definition of thermodynamic temperature
Thermodynamic temperature
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
starting with a null point of absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
. A scale for thermodynamic temperature is established similarly to the empirical temperature scales, however, needing only one additional fixing point.
Empirical scales
Empirical scales are based on the measurement of physical parameters that express the property of interest to be measured trough some formal, most commonly a simple linear, functional relationship. For the measurement of temperature, the formal definition of thermal equilibrium in terms of the thermodynamic coordinate spaces of thermodynamic systems, expressed in the zeroth law of thermodynamicsZeroth law of thermodynamics
The zeroth law of thermodynamics is a generalization principle of thermal equilibrium among bodies, or thermodynamic systems, in contact.The zeroth law states that if two systems are in thermal equilibrium with a third system, they are also in thermal equilibrium with each other.Systems are said to...
, provides the framework to measure temperature.
All temperature scales, including the modern thermodynamic temperature scale used in the International System of Units
International System of Units
The International System of Units is the modern form of the metric system and is generally a system of units of measurement devised around seven base units and the convenience of the number ten. The older metric system included several groups of units...
, are calibrated according to thermal properties of a particular substance or device. Typically, this is established by fixing two well-defined temperature points and defining temperature increments via a linear function of the response of the thermometric device. For example, both the old Celsius scale and Fahrenheit scale were originally based on the linear expansion of a narrow mercury column within a limited range of temperature., each using different reference points and scale increments.
Different empirical scales may not be compatible with each other, except for small regions of temperature overlap. If an alcohol thermometer
Thermometer
Developed during the 16th and 17th centuries, a thermometer is a device that measures temperature or temperature gradient using a variety of different principles. A thermometer has two important elements: the temperature sensor Developed during the 16th and 17th centuries, a thermometer (from the...
and a mercury thermometer
Thermometer
Developed during the 16th and 17th centuries, a thermometer is a device that measures temperature or temperature gradient using a variety of different principles. A thermometer has two important elements: the temperature sensor Developed during the 16th and 17th centuries, a thermometer (from the...
have same two fixed points, namely the freezing and boiling point of water, their reading will not agree with each other except at the fixed points, as the linear 1:1 relationship of expansion between any two thermometric substances may not be guaranteed.
Empirical temperature scales are not reflective of the fundamental, microscopic laws of matter. Temperature is a universal attribute of matter, yet empirical scales map a narrow range onto a scale that is known to have a useful functional form for a particular application. Thus, their range is limited. The working material only exists in a form under certain circumstances, beyond which it no longer can serve as a scale. For example, mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
freezes below 234.32 K, so temperature lower than that cannot be measured in a scale based on mercury. Even ITS-90, which interpolates among different ranges of temperature, has only a range of 0.65 K to approximately 1358 K (−272.5 °C to 1085 °C).
Ideal gas scale
When pressure approaches zero, all real gas will behave like ideal gas, that is, of a mole of gas relying only on temperature. Therefore we can design a scale with as its argument. Of course any bijective function will do, but for convenience's sake linear function is the best. Therefore we define it asThe ideal gas scale is in some sense a "mixed" scale. It relies on the universal properties of gas, a big advance from just a particular substance. But still it is empirical since it puts gas at a special position and thus has limited applicability—at some point no gas can exist. One distinguishing characteristic of ideal gas scale, however, is that it precisely equals thermodynamical scale when it is well defined (see below).
International temperature scale of 1990
ITS-90 is designed to represent the thermodynamic temperature scale (referencing absolute zeroAbsolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
) as closely as possible throughout its range. Many different thermometer designs are required to cover the entire range. These include helium vapor pressure thermometers, helium gas thermometers, standard platinum resistance thermometers
Resistance thermometer
Resistance thermometers, also called resistance temperature detectors or resistive thermal devices , are sensors used to measure temperature by correlating the resistance of the RTD element with temperature. Most RTD elements consist of a length of fine coiled wire wrapped around a ceramic or glass...
(known as SPRTs, PRTs or Platium RTDs) and monochromatic radiation thermometers
Infrared thermometer
Infrared thermometers infer temperature using a portion of the thermal radiation sometimes called blackbody radiation emitted by the object of measurement. They are sometimes called laser thermometers if a laser is used to help aim the thermometer, or non-contact thermometers to describe the...
.
Although the Kelvin and Celsius scales are defined using absolute zero (0 K) and the triple point
Triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium...
of water (273.16 K and 0.01 °C), it is impractical to use this definition at temperatures that are very different from the triple point of water. Accordingly, ITS–90 uses numerous defined points, all of which are based on various thermodynamic equilibrium states of fourteen pure chemical elements and one compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
(water). Most of the defined points are based on a phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
; specifically the melting
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
/freezing
Freezing
Freezing or solidification is a phase change in which a liquid turns into a solid when its temperature is lowered below its freezing point. The reverse process is melting....
point of a pure chemical element. However, the deepest cryogenic
Cryogenics
In physics, cryogenics is the study of the production of very low temperature and the behavior of materials at those temperatures. A person who studies elements under extremely cold temperature is called a cryogenicist. Rather than the relative temperature scales of Celsius and Fahrenheit,...
points are based exclusively on the vapor pressure
Vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed system. All liquids have a tendency to evaporate, and some solids can sublimate into a gaseous form...
/temperature relationship of helium and its isotopes whereas the remainder of its cold points (those less than room temperature) are based on triple point
Triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium...
s. Examples of other defining points are the triple point of hydrogen (−259.3467 °C) and the freezing point of aluminum (660.323 °C).
Thermometers calibrated per ITS–90 use complex mathematical formulas to interpolate between its defined points. ITS–90 specifies rigorous control over variables to ensure reproducibility from lab to lab. For instance, the small effect that atmospheric pressure has upon the various melting points is compensated for (an effect that typically amounts to no more than half a millikelvin across the different altitudes and barometric pressures likely to be encountered). The standard even compensates for the pressure effect due to how deeply the temperature probe is immersed into the sample. ITS–90 also draws a distinction between “freezing” and “melting” points. The distinction depends on whether heat is going into (melting) or out of (freezing) the sample when the measurement is made. Only gallium is measured while melting, all the other metals are measured while the samples are freezing.
There are often small differences between measurements calibrated per ITS–90 and thermodynamic temperature. For instance, precise measurements show that the boiling point of VSMOW
VSMOW
Vienna Standard Mean Ocean Water is a water standard defining the isotopic composition of water. It was promulgated by the International Atomic Energy Agency in 1968....
water under one standard atmosphere of pressure is actually 373.1339 K (99.9839 °C) when adhering strictly to the two-point definition of thermodynamic temperature. When calibrated to ITS–90, where one must interpolate between the defining points of gallium and indium, the boiling point of VSMOW water is about 10 mK less, about 99.974 °C. The virtue of ITS–90 is that another lab in another part of the world will measure the very same temperature with ease due to the advantages of a comprehensive international calibration standard featuring many conveniently spaced, reproducible, defining points spanning a wide range of temperatures.
Celsius scale
Celsius (known until 1948 as centigrade) is a temperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
scale that is named after the Swedish astronomer Anders Celsius
Anders Celsius
Anders Celsius was a Swedish astronomer. He was professor of astronomy at Uppsala University from 1730 to 1744, but traveled from 1732 to 1735 visiting notable observatories in Germany, Italy and France. He founded the Uppsala Astronomical Observatory in 1741, and in 1742 he proposed the Celsius...
(1701–1744), who developed a similar temperature scale two years before his death. The degree Celsius (°C) can refer to a specific temperature on the Celsius scale as well as a unit to indicate a temperature interval
Interval (mathematics)
In mathematics, a interval is a set of real numbers with the property that any number that lies between two numbers in the set is also included in the set. For example, the set of all numbers satisfying is an interval which contains and , as well as all numbers between them...
(a difference between two temperatures or an uncertainty
Uncertainty
Uncertainty is a term used in subtly different ways in a number of fields, including physics, philosophy, statistics, economics, finance, insurance, psychology, sociology, engineering, and information science...
).
From 1744 until 1954, 0 °C was defined as the freezing point of water and 100 °C was defined as the boiling point of water, both at a pressure of one standard atmosphere
Atmosphere (unit)
The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa...
. Although these defining correlations are commonly taught in schools today, by international agreement the unit "degree Celsius" and the Celsius scale are currently defined by two different points: absolute zero
Absolute zero
Absolute zero is the theoretical temperature at which entropy reaches its minimum value. The laws of thermodynamics state that absolute zero cannot be reached using only thermodynamic means....
, and the triple point
Triple point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium...
of VSMOW (specially prepared water). This definition also precisely relates the Celsius scale to the Kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
scale, which defines the SI
Si
Si, si, or SI may refer to :- Measurement, mathematics and science :* International System of Units , the modern international standard version of the metric system...
base unit
SI base unit
The International System of Units defines seven units of measure as a basic set from which all other SI units are derived. These SI base units and their physical quantities are:* metre for length...
of thermodynamic temperature
Thermodynamic temperature
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
(symbol: K). Absolute zero, the hypothetical but unattainable temperature at which matter exhibits zero entropy, is defined as being precisely 0 K and −273.15 °C. The temperature value of the triple point of water is defined as being precisely 273.16 K and 0.01 °C.
This definition fixes the magnitude of both the degree Celsius and the kelvin as precisely 1 part in 273.16 parts, the difference between absolute zero and the triple point of water. Thus, it sets the magnitude of one degree Celsius and that of one kelvin as exactly the same. Additionally, it establishes the difference between the two scales' null points as being precisely 273.15 degrees Celsius (−273.15 °C = 0 K and 0 °C = 273.15 K).
Thermodynamic scale
Thermodynamic scale differs from empirical scales in that it is absolute. It is based on the fundamental laws of thermodynamics or statistical mechanics instead of some arbitrary chosen working material. Besides it covers full range of temperature and has simple relation with microscopic quantities like the average kinetic energy of particles (see equipartition theoremEquipartition theorem
In classical statistical mechanics, the equipartition theorem is a general formula that relates the temperature of a system with its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition...
). In experiments ITS-90 is used to approximate thermodynamic scale due to simpler realization.
Definition
Lord Kelvin devised the thermodynamic scale based on the efficiency of heat engines as shown below:The efficiency of a engine is the work divided by the heat introduced to the system or
-
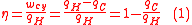 ,
,
where wcy is the work done per cycle. Thus, the efficiency depends only on qC/qH.
Because of Carnot theorem, any reversible heat engine operating between temperatures T1 and T2 must have the same efficiency, meaning, the efficiency is the function of the temperatures only:
In addition, a reversible heat engine operating between temperatures T1 and T3 must have the same efficiency as one consisting of two cycles, one between T1 and another (intermediate) temperature T2, and the second between T2andT3. This can only be the case if
-
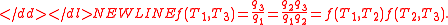
Specializing to the case that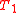 is a fixed reference temperature: the temperature of the triple point of water. Then for anyT2and T3,
is a fixed reference temperature: the temperature of the triple point of water. Then for anyT2and T3,
Therefore, if thermodynamic temperature is defined by
then the function f, viewed as a function of thermodynamic temperature, is
and the reference temperature T1 has the value 273.16. (Of course any reference temperature and any positive numerical value could be used—the choice here corresponds to the KelvinKelvinThe kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
scale.)
Equality to ideal gas scale
It follows immediately that
Substituting Equation 3 back into Equation 1 gives a relationship for the efficiency in terms of temperature:
This is identical to the efficiency formula for Carnot cycleCarnot cycleThe Carnot cycle is a theoretical thermodynamic cycle proposed by Nicolas Léonard Sadi Carnot in 1824 and expanded by Benoit Paul Émile Clapeyron in the 1830s and 40s. It can be shown that it is the most efficient cycle for converting a given amount of thermal energy into work, or conversely,...
, which effectively employs the ideal gas scale. So this means the two scales equals numerically at every point.
See also
- Temperature conversion
-