
Sesquiterpene
Encyclopedia
Sesquiterpenes are a class of terpene
s that consist of three isoprene
units and have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be acyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation
or rearrangement
produce the related sesquiterpenoids.
Sesquiterpenes are found naturally in plants and insects, as semiochemical
s, e.g. defensive agents or pheromones.
reacts with isopentenyl pyrophosphate
, the result is the 15-carbon farnesyl pyrophosphate
, which is an intermediate in the biosynthesis of sesquiterpenes such as farnesene
. Oxidation can then provide sesquiterpenoids such as farnesol
.

, a constituent of the oil from ginger
, cyclization of one end of the chain to the other end can lead to macrocyclic rings such as humulene
.
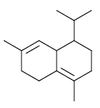 In addition to common six-membered rings such as in the cadinene
In addition to common six-membered rings such as in the cadinene
s, one classic bicyclic sesquiterpene is caryophyllene
, from the oil of clove
s, which has a nine-membered ring and cyclobutane
ring. Additional unsaturation provides aromatic bicyclic sesquiterpenoids such as vetivazulene
and guaiazulene
.
, copaene
and the alcohol patchoulol
.
Terpene
Terpenes are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers, though also by some insects such as termites or swallowtail butterflies, which emit terpenes from their osmeterium. They are often strong smelling and thus may have had a protective...
s that consist of three isoprene
Isoprene
Isoprene , or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=CCH=CH2. Under standard conditions it is a colorless liquid...
units and have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be acyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
or rearrangement
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
produce the related sesquiterpenoids.
Sesquiterpenes are found naturally in plants and insects, as semiochemical
Semiochemical
A semiochemical is a generic term used for a chemical substance or mixture that carries a message. These chemicals acts as messengers within or between species...
s, e.g. defensive agents or pheromones.
Acyclic
When geranyl pyrophosphateGeranyl pyrophosphate
Geranyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of farnesyl pyrophosphate, geranylgeranyl pyrophosphate, cholesterol, terpenes and terpenoids....
reacts with isopentenyl pyrophosphate
Isopentenyl pyrophosphate
Isopentenyl pyrophosphate is an intermediate in the classical, HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes and terpenoids. IPP is formed from acetyl-CoA via mevalonic acid...
, the result is the 15-carbon farnesyl pyrophosphate
Farnesyl pyrophosphate
Farnesyl pyrophosphate is an intermediate in the HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes, terpenoids, and sterols...
, which is an intermediate in the biosynthesis of sesquiterpenes such as farnesene
Farnesene
The term farnesene refers to a set of six closely related chemical compounds which all are sesquiterpenes. α-Farnesene and β-farnesene are isomers, differing by the location of one double bond. α-Farnesene is 3,7,11-trimethyl-1,3,6,10-dodecatetraene and β-farnesene is...
. Oxidation can then provide sesquiterpenoids such as farnesol
Farnesol
Farnesol is a natural organic compound which is an acyclic sesquiterpene alcohol found as a colorless liquid. It is insoluble in water, but miscible with oils...
.

Monocyclic
With the increased chain length and additional double bond, the number of possible ways that cyclization can occur is also increased, and there exists a wide variety of cyclic sesquiterpenes. In addition to common six-membered ring systems such as is found in zingibereneZingiberene
Zingiberene is a monocyclic sesquiterpene that is the predominant constituent of the oil of ginger , from which it gets its name....
, a constituent of the oil from ginger
Ginger
Ginger is the rhizome of the plant Zingiber officinale, consumed as a delicacy, medicine, or spice. It lends its name to its genus and family . Other notable members of this plant family are turmeric, cardamom, and galangal....
, cyclization of one end of the chain to the other end can lead to macrocyclic rings such as humulene
Humulene
Humulene, also known as α-humulene or α-caryophyllene, is a naturally occurring monocyclic sesquiterpene, which is a terpenoid consisting of 3 isoprene units. It is found in the essential oils of Humulus lupulus from which it derives its name. It is an isomer of β-caryophyllene, and the two are...
.
Bicyclic

Cadinene
Cadinene is the trivial chemical name of a number of isomeric hydrocarbons that occur in a wide variety of essential oil-producing plants. The name is derived from that of the Cade juniper , the wood of which yields an oil from which cadinene isomers were first isolated.Chemically, the cadinenes...
s, one classic bicyclic sesquiterpene is caryophyllene
Caryophyllene
Caryophyllene , or -β-caryophyllene, is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum , the essential oil of hemp Cannabis sativa, rosemary Rosmarinus oficinalis, and hops...
, from the oil of clove
Clove
Cloves are the aromatic dried flower buds of a tree in the family Myrtaceae. Cloves are native to the Maluku islands in Indonesia and used as a spice in cuisines all over the world...
s, which has a nine-membered ring and cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
ring. Additional unsaturation provides aromatic bicyclic sesquiterpenoids such as vetivazulene
Vetivazulene
Vetivazulene is an azulene derivate obtained from vetiver oil. It is a bicyclic sesquiterpene and an isomer of guaiazulene....
and guaiazulene
Guaiazulene
Guaiazulene, also azulon or 1,4-dimethyl-7-isopropylazulene, is a dark blue crystalline hydrocarbon. A derivative of azulene, guaiazulene is a bicyclic sesquiterpene that is a constituent of some essential oils, mainly oil of guaiac and chamomile oil, which also serve as its commercial sources....
.
Tricyclic
With the addition of a third ring, the possible structures become increasingly varied. Examples include longifoleneLongifolene
Longifolene is the common chemical name of a naturally occurring, oily liquid hydrocarbon found primarily in the high-boiling fraction of certain pine resins. The name is derived from that of a pine species from which the compound was isolated, Pinus longifolia Chemically, longifolene is a...
, copaene
Copaene
Copaene, or more precisely, α-copaene, is the common chemical name of an oily liquid hydrocarbon that is found in a number of essential oil-producing plants. The name is derived from that of the resin-producing tropical copaiba tree, Copaifera langsdorfii, from which the compound was first...
and the alcohol patchoulol
Patchoulol
Patchoulol or patchouli alcohol is a terpene extracted from Patchouli. The -optical isomer is one of the organic compounds responsible for the typical patchouli scent. Patchoulol is also used in the synthesis of the chemotherapy drug Taxol....
.

