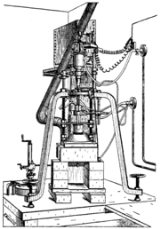
Verneuil process
Encyclopedia
The Verneuil process, also called flame fusion, was the first commercially successful method of manufacturing synthetic gemstone
s, developed in 1902 by the French
chemist Auguste Verneuil
. It is primarily used to produce the ruby
and sapphire
varieties of corundum
, as well as the diamond simulant
s rutile
and strontium titanate
. The principle of the process involves melting a finely powdered substance using an oxyhydrogen flame, and crystallising the melted droplets into a boule
. The process is considered to be the founding step of modern industrial crystal growth
technology, and remains in wide use to this day.
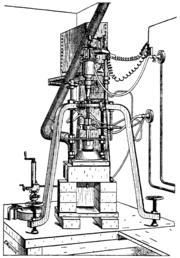 Since the time of the alchemists
Since the time of the alchemists
, there have been attempts to synthetically produce precious stones, and ruby
, being one of the five highly prized cardinal gem
s, has long been a prime candidate for synthesis. In the 19th century, those attempts became successful, with the first ruby produced by melting two smaller rubies together in 1817, and the first microscopic crystals created from alumina (aluminium oxide
) in a laboratory in 1837. By 1877, chemist Edmond Fremy
had devised an effective method for commercial ruby manufacture by using molten baths of alumina, yielding the first gemstone-quality synthetic stones. The Paris
ian chemist Auguste Verneuil collaborated with Fremy on developing the method, but soon went on to independently develop the flame fusion process, which would eventually come to bear his name.
One of Verneuil's sources of inspiration for developing his own method was the appearance of synthetic rubies sold by an unknown Geneva
n merchant in 1880. These "Geneva rubies" were dismissed as artificial at the time, but are now believed to be the first rubies produced by flame fusion, predating Verneuil's work on the process by 20 years. After examining the "Geneva rubies", Verneuil came to the conclusion that it was possible to recrystallise finely ground aluminium oxide into a large gemstone. This realisation, along with the availability of the recently developed oxyhydrogen torch and growing demand for synthetic rubies, led him to design the Verneuil furnace, where finely ground purified alumina and chromium oxide
were melted by a flame of at least 2000 °C (3,600 °F), and recrystallised on a support below the flame, creating a large crystal. He announced his work in 1902, publishing details outlining the process in 1904.
By 1910, Verneuil's laboratory had expanded into a 30 furnace production facility, with annual gemstone production by the Verneuil process having reached 1,000 kg (2,205 lb) in 1907. By 1912, production reached 3,200 kg (7,100 lb) , and would go on to reach 200,000 kg (440,000 lb) in 1980 and 250,000 kg (550,000 lb) in 2000, led by Hrand Djevahirdjian's factory in Monthey
, Switzerland
, founded in 1914. The most notable improvements in the process were made in 1932, by S. K. Popov, who helped establish the capability for producing high-quality sapphires in the Soviet Union
through the next 20 years. A large production capability was also established in the United States
during World War II
, when European sources were not available, and jewels were in high demand for their military applications.
The process was designed primarily for the synthesis of rubies, which became the first gemstones to be synthetically produced, thanks to the efforts of Fremy and Verneuil. However, the Verneuil process could also be used for the production of other stones, including blue sapphire, which simply required ferric oxide
to be substituted for chromium oxide, as well as more elaborate ones, such as star sapphires, where titania (titanium dioxide
) was added and the boule was kept in the heat longer, allowing needles of rutile
to crystallise within it. In 1947, the Linde Air Products division of Union Carbide
, pioneered the use of the Verneuil process for creating such star sapphires, until production was discontinued in 1974 due to overseas competition.
Despite some improvements in the method, the Verneuil process remains virtually unchanged to this day, while maintaining a leading position in the manufacture of synthetic corundum and spinel
gemstones. Its most significant setback came in 1917, when Jan Czochralski
introduced the Czochralski process
, which has found numerous applications in the semiconductor industry
, where a much higher quality of crystals is required than the Verneuil process can produce. Other alternatives to the process emerged in 1957, when Bell Labs
introduced the hydrothermal process
, and in 1958, when Carroll Chatham introduced the flux process. In 1989 Larry P Kelley of ICT, Inc. also developed a variant of the Czochralski process where natural ruby is used as the 'feed' material.
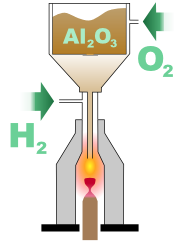
 One of the most crucial factors in successfully crystallising an artificial gemstone is obtaining highly pure starting material, with at least 99.9995% purity. In the case of manufacturing rubies or sapphires, this material is alumina. The presence of sodium
One of the most crucial factors in successfully crystallising an artificial gemstone is obtaining highly pure starting material, with at least 99.9995% purity. In the case of manufacturing rubies or sapphires, this material is alumina. The presence of sodium
impurities is especially undesirable, as it makes the crystal opaque
. Depending on the desired colouration of the crystal, small quantities of various oxide
s are added, such as chromium oxide for a red ruby, or ferric oxide and titania for a blue sapphire. Other starting materials include titania for producing rutile, or titanyl double oxalate
for producing strontium titanate. Alternatively, small, valueless crystals of the desired product can be used.
This starting material is finely powdered, and placed in a container within a Verneuil furnace, with an opening at the bottom through which the powder can escape when the container is vibrated. While the powder is being released, oxygen
is supplied into the furnace, and travels with the powder down a narrow tube. This tube is located within a larger tube, into which hydrogen
is supplied. At the point where the narrow tube opens into the larger one, combustion
occurs, with a flame of at least 2000 °C (3,600 °F) at its core. As the powder passes through the flame, it melts into small droplets, which fall onto an earthen support rod placed below. The droplets gradually form a sinter
cone on the rod, the tip of which is close enough to the core to remain liquid. It is at that tip that the seed crystal
eventually forms. As more droplets fall onto the tip, a single crystal
, called a boule, starts to form, and the support is slowly moved downward, allowing the base of the boule to crystallise, while its cap always remains liquid. The boule is formed in the shape of a tapered cylinder, with a diameter broadening away from the base and eventually remaining more or less constant. With a constant supply of powder and withdrawal of the support, very long cylindrical boules can be obtained. Once removed from the furnace and allowed to cool, the boule is split along its vertical axis to relieve internal pressure, otherwise the crystal will be prone to fracture when the stalk is broken due to a vertical parting plane
.
When initially outlining the process, Verneuil specified a number of conditions crucial for good results. These include: a flame temperature that is not higher than necessary for fusion; always keeping the melted product in the same part of the oxyhydrogen flame; and reducing the point of contact between the melted product and support to as small an area as possible. The average commercially produced boule using the process is 13 mm (0.5 inches) in diameter and 25 to 50 mm (1 to 2 inches) long, weighing about 125 carats (25 g). The process can also be performed with a custom-oriented seed crystal to achieve a specific desired crystallographic orientation
.
Crystals produced by the Verneuil process are chemically and physically equivalent to their naturally occurring counterparts, and strong magnification is usually required to distinguish between the two. One of the telltale characteristics of a Verneuil crystal is curved growth lines (curved striae) formed as the cylindrical boule grows upwards in an environment with a high thermal gradient; the equivalent lines in natural crystals are parallel. Another distinguishing feature is the common presence of microscopic gas bubbles formed due to an excess of oxygen in the furnace; imperfections in natural crystals are usually solid impurities.
Gemstone
A gemstone or gem is a piece of mineral, which, in cut and polished form, is used to make jewelry or other adornments...
s, developed in 1902 by the French
French people
The French are a nation that share a common French culture and speak the French language as a mother tongue. Historically, the French population are descended from peoples of Celtic, Latin and Germanic origin, and are today a mixture of several ethnic groups...
chemist Auguste Verneuil
Auguste Victor Louis Verneuil
Auguste Victor Louis Verneuil was a French chemist best known for inventing the first commercially viable process for the manufacture of synthetic gemstones...
. It is primarily used to produce the ruby
Ruby
A ruby is a pink to blood-red colored gemstone, a variety of the mineral corundum . The red color is caused mainly by the presence of the element chromium. Its name comes from ruber, Latin for red. Other varieties of gem-quality corundum are called sapphires...
and sapphire
Sapphire
Sapphire is a gemstone variety of the mineral corundum, an aluminium oxide , when it is a color other than red or dark pink; in which case the gem would instead be called a ruby, considered to be a different gemstone. Trace amounts of other elements such as iron, titanium, or chromium can give...
varieties of corundum
Corundum
Corundum is a crystalline form of aluminium oxide with traces of iron, titanium and chromium. It is a rock-forming mineral. It is one of the naturally clear transparent materials, but can have different colors when impurities are present. Transparent specimens are used as gems, called ruby if red...
, as well as the diamond simulant
Diamond simulant
The high price of gem-grade diamonds, as well as significant ethical concerns of the diamond trade, have created a large demand for materials with similar gemological characteristics, known as diamond simulants or imitations. Simulants are distinct from synthetic diamond, which unlike simulants is...
s rutile
Rutile
Rutile is a mineral composed primarily of titanium dioxide, TiO2.Rutile is the most common natural form of TiO2. Two rarer polymorphs of TiO2 are known:...
and strontium titanate
Strontium titanate
Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure...
. The principle of the process involves melting a finely powdered substance using an oxyhydrogen flame, and crystallising the melted droplets into a boule
Boule (crystal)
A boule is a single-crystal ingot produced by synthetic means. A boule of silicon is the starting material for most of the integrated circuits used today....
. The process is considered to be the founding step of modern industrial crystal growth
Crystal growth
A crystal is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process, and consists in the addition of new atoms, ions, or polymer strings into...
technology, and remains in wide use to this day.
History

Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
, there have been attempts to synthetically produce precious stones, and ruby
Ruby
A ruby is a pink to blood-red colored gemstone, a variety of the mineral corundum . The red color is caused mainly by the presence of the element chromium. Its name comes from ruber, Latin for red. Other varieties of gem-quality corundum are called sapphires...
, being one of the five highly prized cardinal gem
Cardinal gem
Cardinal gems are gems which have traditionally considered precious above all others. The classification of the cardinal gems dates back to antiquity, and was largely determined by ceremonial or religious use and rarity. The term has largely fallen out of favour with modern gemologists.The five...
s, has long been a prime candidate for synthesis. In the 19th century, those attempts became successful, with the first ruby produced by melting two smaller rubies together in 1817, and the first microscopic crystals created from alumina (aluminium oxide
Aluminium oxide
Aluminium oxide is an amphoteric oxide with the chemical formula 23. It is commonly referred to as alumina, or corundum in its crystalline form, as well as many other names, reflecting its widespread occurrence in nature and industry...
) in a laboratory in 1837. By 1877, chemist Edmond Fremy
Edmond Fremy
Edmond Frémy was a French chemist. He is perhaps best known today for Frémy's salt, a strong oxidizing agent which he discovered in 1845...
had devised an effective method for commercial ruby manufacture by using molten baths of alumina, yielding the first gemstone-quality synthetic stones. The Paris
Paris
Paris is the capital and largest city in France, situated on the river Seine, in northern France, at the heart of the Île-de-France region...
ian chemist Auguste Verneuil collaborated with Fremy on developing the method, but soon went on to independently develop the flame fusion process, which would eventually come to bear his name.
One of Verneuil's sources of inspiration for developing his own method was the appearance of synthetic rubies sold by an unknown Geneva
Geneva
Geneva In the national languages of Switzerland the city is known as Genf , Ginevra and Genevra is the second-most-populous city in Switzerland and is the most populous city of Romandie, the French-speaking part of Switzerland...
n merchant in 1880. These "Geneva rubies" were dismissed as artificial at the time, but are now believed to be the first rubies produced by flame fusion, predating Verneuil's work on the process by 20 years. After examining the "Geneva rubies", Verneuil came to the conclusion that it was possible to recrystallise finely ground aluminium oxide into a large gemstone. This realisation, along with the availability of the recently developed oxyhydrogen torch and growing demand for synthetic rubies, led him to design the Verneuil furnace, where finely ground purified alumina and chromium oxide
Chromium oxide
Chromium oxide may refer to:* Chromium oxide, CrO* Chromium oxide, Cr2O3* Chromium dioxide , CrO2* Chromium trioxide , CrO3* Chromium oxide peroxide, CrO5* Mixed valence species, such as Cr8O21...
were melted by a flame of at least 2000 °C (3,600 °F), and recrystallised on a support below the flame, creating a large crystal. He announced his work in 1902, publishing details outlining the process in 1904.
By 1910, Verneuil's laboratory had expanded into a 30 furnace production facility, with annual gemstone production by the Verneuil process having reached 1,000 kg (2,205 lb) in 1907. By 1912, production reached 3,200 kg (7,100 lb) , and would go on to reach 200,000 kg (440,000 lb) in 1980 and 250,000 kg (550,000 lb) in 2000, led by Hrand Djevahirdjian's factory in Monthey
Monthey
Monthey is the capital of the district of Monthey in the canton of Valais in Switzerland.- History :The castle in the town center was built in 950 on a hill, the first houses of Monthey surrounded it. Monthey is first mentioned in 1215 as Montez At the 13th century, the counts of Savoy owned the...
, Switzerland
Switzerland
Switzerland name of one of the Swiss cantons. ; ; ; or ), in its full name the Swiss Confederation , is a federal republic consisting of 26 cantons, with Bern as the seat of the federal authorities. The country is situated in Western Europe,Or Central Europe depending on the definition....
, founded in 1914. The most notable improvements in the process were made in 1932, by S. K. Popov, who helped establish the capability for producing high-quality sapphires in the Soviet Union
Soviet Union
The Soviet Union , officially the Union of Soviet Socialist Republics , was a constitutionally socialist state that existed in Eurasia between 1922 and 1991....
through the next 20 years. A large production capability was also established in the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
during World War II
World War II
World War II, or the Second World War , was a global conflict lasting from 1939 to 1945, involving most of the world's nations—including all of the great powers—eventually forming two opposing military alliances: the Allies and the Axis...
, when European sources were not available, and jewels were in high demand for their military applications.
The process was designed primarily for the synthesis of rubies, which became the first gemstones to be synthetically produced, thanks to the efforts of Fremy and Verneuil. However, the Verneuil process could also be used for the production of other stones, including blue sapphire, which simply required ferric oxide
Iron(III) oxide
Iron oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron oxide , which is rare, and iron oxide , which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main...
to be substituted for chromium oxide, as well as more elaborate ones, such as star sapphires, where titania (titanium dioxide
Titanium dioxide
Titanium dioxide, also known as titanium oxide or titania, is the naturally occurring oxide of titanium, chemical formula . When used as a pigment, it is called titanium white, Pigment White 6, or CI 77891. Generally it comes in two different forms, rutile and anatase. It has a wide range of...
) was added and the boule was kept in the heat longer, allowing needles of rutile
Rutile
Rutile is a mineral composed primarily of titanium dioxide, TiO2.Rutile is the most common natural form of TiO2. Two rarer polymorphs of TiO2 are known:...
to crystallise within it. In 1947, the Linde Air Products division of Union Carbide
Union Carbide
Union Carbide Corporation is a wholly owned subsidiary of The Dow Chemical Company. It currently employs more than 2,400 people. Union Carbide primarily produces chemicals and polymers that undergo one or more further conversions by customers before reaching consumers. Some are high-volume...
, pioneered the use of the Verneuil process for creating such star sapphires, until production was discontinued in 1974 due to overseas competition.
Despite some improvements in the method, the Verneuil process remains virtually unchanged to this day, while maintaining a leading position in the manufacture of synthetic corundum and spinel
Spinel
Spinel is the magnesium aluminium member of the larger spinel group of minerals. It has the formula MgAl2O4. Balas ruby is an old name for a rose-tinted variety.-Spinel group:...
gemstones. Its most significant setback came in 1917, when Jan Czochralski
Jan Czochralski
Jan Czochralski was a Polish chemist who invented the Czochralski process, which is used to grow single crystals and is used in the production of semiconductor wafers....
introduced the Czochralski process
Czochralski process
The Czochralski process is a method of crystal growth used to obtain single crystals of semiconductors , metals , salts, and synthetic gemstones...
, which has found numerous applications in the semiconductor industry
Semiconductor industry
The semiconductor industry is the aggregate collection of companies engaged in the design and fabrication of semiconductor devices. It formed around 1960, once the fabrication of semiconductors became a viable business...
, where a much higher quality of crystals is required than the Verneuil process can produce. Other alternatives to the process emerged in 1957, when Bell Labs
Bell Labs
Bell Laboratories is the research and development subsidiary of the French-owned Alcatel-Lucent and previously of the American Telephone & Telegraph Company , half-owned through its Western Electric manufacturing subsidiary.Bell Laboratories operates its...
introduced the hydrothermal process
Hydrothermal synthesis
Hydrothermal synthesis includes the various techniques of crystallizing substances from high-temperature aqueous solutions at high vapor pressures; also termed "hydrothermal method". The term "hydrothermal" is of geologic origin. Geochemists and mineralogists have studied hydrothermal phase...
, and in 1958, when Carroll Chatham introduced the flux process. In 1989 Larry P Kelley of ICT, Inc. also developed a variant of the Czochralski process where natural ruby is used as the 'feed' material.
Process


Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
impurities is especially undesirable, as it makes the crystal opaque
Opacity (optics)
Opacity is the measure of impenetrability to electromagnetic or other kinds of radiation, especially visible light. In radiative transfer, it describes the absorption and scattering of radiation in a medium, such as a plasma, dielectric, shielding material, glass, etc...
. Depending on the desired colouration of the crystal, small quantities of various oxide
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
s are added, such as chromium oxide for a red ruby, or ferric oxide and titania for a blue sapphire. Other starting materials include titania for producing rutile, or titanyl double oxalate
Oxalate
Oxalate , is the dianion with formula C2O42− also written 22−. Either name is often used for derivatives, such as disodium oxalate, 2C2O42−, or an ester of oxalic acid Oxalate (IUPAC: ethanedioate), is the dianion with formula C2O42− also written (COO)22−. Either...
for producing strontium titanate. Alternatively, small, valueless crystals of the desired product can be used.
This starting material is finely powdered, and placed in a container within a Verneuil furnace, with an opening at the bottom through which the powder can escape when the container is vibrated. While the powder is being released, oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
is supplied into the furnace, and travels with the powder down a narrow tube. This tube is located within a larger tube, into which hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
is supplied. At the point where the narrow tube opens into the larger one, combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
occurs, with a flame of at least 2000 °C (3,600 °F) at its core. As the powder passes through the flame, it melts into small droplets, which fall onto an earthen support rod placed below. The droplets gradually form a sinter
Sintering
Sintering is a method used to create objects from powders. It is based on atomic diffusion. Diffusion occurs in any material above absolute zero, but it occurs much faster at higher temperatures. In most sintering processes, the powdered material is held in a mold and then heated to a temperature...
cone on the rod, the tip of which is close enough to the core to remain liquid. It is at that tip that the seed crystal
Seed crystal
A seed crystal is a small piece of single crystal/polycrystal material from which a large crystal of the same material typically is to be grown...
eventually forms. As more droplets fall onto the tip, a single crystal
Single crystal
A single crystal or monocrystalline solid is a material in which the crystal lattice of the entire sample is continuous and unbroken to the edges of the sample, with no grain boundaries...
, called a boule, starts to form, and the support is slowly moved downward, allowing the base of the boule to crystallise, while its cap always remains liquid. The boule is formed in the shape of a tapered cylinder, with a diameter broadening away from the base and eventually remaining more or less constant. With a constant supply of powder and withdrawal of the support, very long cylindrical boules can be obtained. Once removed from the furnace and allowed to cool, the boule is split along its vertical axis to relieve internal pressure, otherwise the crystal will be prone to fracture when the stalk is broken due to a vertical parting plane
Cleavage (crystal)
Cleavage, in mineralogy, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the...
.
When initially outlining the process, Verneuil specified a number of conditions crucial for good results. These include: a flame temperature that is not higher than necessary for fusion; always keeping the melted product in the same part of the oxyhydrogen flame; and reducing the point of contact between the melted product and support to as small an area as possible. The average commercially produced boule using the process is 13 mm (0.5 inches) in diameter and 25 to 50 mm (1 to 2 inches) long, weighing about 125 carats (25 g). The process can also be performed with a custom-oriented seed crystal to achieve a specific desired crystallographic orientation
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
.
Crystals produced by the Verneuil process are chemically and physically equivalent to their naturally occurring counterparts, and strong magnification is usually required to distinguish between the two. One of the telltale characteristics of a Verneuil crystal is curved growth lines (curved striae) formed as the cylindrical boule grows upwards in an environment with a high thermal gradient; the equivalent lines in natural crystals are parallel. Another distinguishing feature is the common presence of microscopic gas bubbles formed due to an excess of oxygen in the furnace; imperfections in natural crystals are usually solid impurities.
External links
- http://www.gemstonebuzz.com/synthesis-synthetic/flame-fusion.html identification of gemstone synthesized by flame fusion.

