
Strontium titanate
Encyclopedia
Strontium titanate is an oxide
of strontium
and titanium
with the chemical formula
Sr
Ti
O
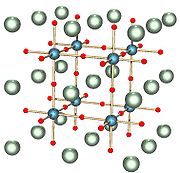
3. At room temperature, it is a centrosymmetric paraelectric
material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia
and named tausonite—was recognised by the IMA
. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystal
s. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant
, in precision optics
, in varistor
s, and in advanced ceramic
s.
The name tausonite was given in honour of Lev Vladimirovich Tauson (1917–1989), a Russian geochemist
. Disused trade names for the synthetic product include strontium mesotitanate, Fabulite, Diagem, and Marvelite. Other than its type locality of the Murun Massif in the Sakha Republic, natural tausonite is also found in Cerro Sarambi, Concepción department
, Paraguay
; and along the Kotaki River of Honshū
, Japan
.

 Strontium titanate is both much denser (specific gravity
Strontium titanate is both much denser (specific gravity
4.88 for natural, 5.13 for synthetic) and much softer (Mohs hardness
6–6.5 for natural, 5.5 for synthetic) than diamond
. Its crystal system
is cubic and its refractive index
(2.41—as measured by sodium
light, 589.3 nm) is nearly identical to that of diamond, but the dispersion
(the optical property responsible for the "fire" of the cut stones) of strontium titanate is over four times higher, at 0.19 (B–G interval). This results in an excess of fire when compared to diamond.
Synthetics are usually transparent and colourless, but can be doped
with certain rare earth
or transition metal
s to give reds, yellows, browns, and blues. Natural tausonite is usually translucent to opaque, in shades of reddish brown, dark red, or grey. Both have an adamantine (diamond-like) lustre
. Strontium titanate is considered extremely brittle with a conchoidal fracture
; natural material is cubic or octahedral in habit
and streaks brown. Through a hand-held (direct vision) spectroscope, doped synthetics will exhibit a rich absorption spectrum typical of doped stones. Synthetic material has a melting point
of ca. 2080°C (3776°F) and is readily attacked by hydrofluoric acid
.
The synthetic material has a very large dielectric constant
(300) at room temperature and low electric field. It is also used in high-voltage capacitors. Strontium titanate becomes superconducting below 0.35 K and was the first insulator and oxide discovered to be superconductive.
At temperatures lower than 105 K, its cubic structure transforms to tetragonal. It is an excellent substrate for epitaxial growth of high-temperature superconductors and many oxide-based thin film
s. Its monocrystals can be used as optical windows and high-quality sputter deposition
targets.
SrTiO3 is a suitable material for electronics: niobium
-doped strontium titanate, is electrically conductive. High-quality, epitaxial SrTiO3 layers can also be grown on silicon
without forming silicon dioxide
, thereby making SrTiO3 an alternative gate dielectric material. This also enables the integration of other thin film perovskite oxides onto silicon.
 Synthetic strontium titanate was one of several titanate
Synthetic strontium titanate was one of several titanate
s patent
ed during the late 1940s and early 1950s; other titanates included barium titanate
and calcium titanate
. Research was conducted primarily at the National Lead Company (later renamed N. L. Industries, Inc.) in the United States
, by Leon Merker and Langtry E. Lynd. Merker and Lynd first patented the growth process on February 10, 1953; a number of refinements were subsequently patented over the next four years, such as modifications to the feed powder and additions of colouring dopants.
A modification to the basic Verneuil process
(also known as flame-fusion) is the favoured method of growth. An inverted oxy-hydrogen blowpipe
is used, with feed powder mixed with oxygen
carefully fed through the blowpipe in the typical fashion, but with the addition of a third pipe to deliver oxygen—creating a tricone burner. The extra oxygen is required for successful formation of strontium titanate, which would otherwise fail to oxidize completely due to the titanium component. The ratio is ca. 1.5 volumes of hydrogen
for each volume of oxygen. The highly purified feed powder is derived by first producing titanyl double oxalate salt
(SrTiO(C
2O4)2·2H2O
) by reacting strontium chloride
(SrCl
2) and oxalic acid
((COOH
)2.2H2O) with titanium tetrachloride
(TiCl4). The salt is washed to completely eliminate chloride
, heated to 1000°C in order to produce a free-flowing granular
powder of the required composition, and is then ground and sieved to ensure all particles are between 0.2–0.5 micrometre
s in size.
The feed powder falls through the oxyhydrogen flame, melts, and lands on a rotating and slowly descending pedestal below. The height of the pedestal is constantly adjusted to keep its top at the optimal position below the flame, and over a number of hours the molten powder cools and crystallises to form a single pedunculated pear or boule
crystal. This boule is usually no larger than 2.5 centimetres in diameter and 10 centimetres long; it is an opaque black to begin with, requiring further annealing
in an oxidizing atmosphere in order to make the crystal colourless and to relieve strain
. This is done at over 1000°C for 12 hours.
("titania") at the time, and had the advantage of lacking the unfortunate yellow tinge and strong birefringence
inherent to the latter material. While it was softer, it was significantly closer to diamond in likeness. Eventually, however, both would fall into disuse, being eclipsed by the creation of "better" simulants: first by yttrium aluminium garnet
(YAG) and followed shortly after by gadolinium gallium garnet
(GGG); and finally by the (to date) ultimate simulant in terms of diamond-likeness and cost-effectiveness, cubic zirconia
.
Despite being outmoded, strontium titanate is still manufactured and periodically encountered in jewellery. It is one of the most costly of diamond simulants, and due to its rarity collectors may pay a premium for large i.e. >2 carat (400 mg) specimens. As a diamond simulant, strontium titanate is most deceptive when mingled with melée i.e. <0.20 carat (40 mg) stones and when it is used as the base material for a composite or doublet stone (with, e.g., synthetic corundum
as the crown or top of the stone). Under the microscope
, gemmologist
s distinguish strontium titanate from diamond by the former's softness—manifested by surface abrasions—and excess dispersion (to the trained eye), and occasional gas bubbles which are remnants of synthesis. Doublets can be detected by a join line at the girdle ("waist" of the stone) and flattened air bubbles or glue visible within the stone at the point of bonding.
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
of strontium
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
and titanium
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
with the chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
Sr
Strontium
Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and...
Ti
Titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color....
O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
3. At room temperature, it is a centrosymmetric paraelectric
Paraelectricity
Paraelectricity is the ability of many materials to become polarized under an applied electric field. Unlike ferroelectricity, this can happen even if there is no permanent electric dipole that exists in the material, and removal of the fields results in the polarization in the material returning...
material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia
Siberia
Siberia is an extensive region constituting almost all of Northern Asia. Comprising the central and eastern portion of the Russian Federation, it was part of the Soviet Union from its beginning, as its predecessor states, the Tsardom of Russia and the Russian Empire, conquered it during the 16th...
and named tausonite—was recognised by the IMA
International Mineralogical Association
The International Mineralogical Association is an international group of 38 national societies. The goal is to promote the science of mineralogy and to standardize the nomenclature of the 4000 plus known mineral species...
. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystal
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
s. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant
Diamond simulant
The high price of gem-grade diamonds, as well as significant ethical concerns of the diamond trade, have created a large demand for materials with similar gemological characteristics, known as diamond simulants or imitations. Simulants are distinct from synthetic diamond, which unlike simulants is...
, in precision optics
Optics
Optics is the branch of physics which involves the behavior and properties of light, including its interactions with matter and the construction of instruments that use or detect it. Optics usually describes the behavior of visible, ultraviolet, and infrared light...
, in varistor
Varistor
A varistor is an electronic component with a "diode-like" nonlinear current–voltage characteristic. The name is a portmanteau of variable resistor...
s, and in advanced ceramic
Ceramic
A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
s.
The name tausonite was given in honour of Lev Vladimirovich Tauson (1917–1989), a Russian geochemist
Geochemistry
The field of geochemistry involves study of the chemical composition of the Earth and other planets, chemical processes and reactions that govern the composition of rocks, water, and soils, and the cycles of matter and energy that transport the Earth's chemical components in time and space, and...
. Disused trade names for the synthetic product include strontium mesotitanate, Fabulite, Diagem, and Marvelite. Other than its type locality of the Murun Massif in the Sakha Republic, natural tausonite is also found in Cerro Sarambi, Concepción department
Concepción Department
Concepción Department may refer to:* Concepción Department * Concepción Department...
, Paraguay
Paraguay
Paraguay , officially the Republic of Paraguay , is a landlocked country in South America. It is bordered by Argentina to the south and southwest, Brazil to the east and northeast, and Bolivia to the northwest. Paraguay lies on both banks of the Paraguay River, which runs through the center of the...
; and along the Kotaki River of Honshū
Honshu
is the largest island of Japan. The nation's main island, it is south of Hokkaido across the Tsugaru Strait, north of Shikoku across the Inland Sea, and northeast of Kyushu across the Kanmon Strait...
, Japan
Japan
Japan is an island nation in East Asia. Located in the Pacific Ocean, it lies to the east of the Sea of Japan, China, North Korea, South Korea and Russia, stretching from the Sea of Okhotsk in the north to the East China Sea and Taiwan in the south...
.
Properties


Specific gravity
Specific gravity is the ratio of the density of a substance to the density of a reference substance. Apparent specific gravity is the ratio of the weight of a volume of the substance to the weight of an equal volume of the reference substance. The reference substance is nearly always water for...
4.88 for natural, 5.13 for synthetic) and much softer (Mohs hardness
Mohs scale of mineral hardness
The Mohs scale of mineral hardness characterizes the scratch resistance of various minerals through the ability of a harder material to scratch a softer material. It was created in 1812 by the German geologist and mineralogist Friedrich Mohs and is one of several definitions of hardness in...
6–6.5 for natural, 5.5 for synthetic) than diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
. Its crystal system
Crystal system
In crystallography, the terms crystal system, crystal family, and lattice system each refer to one of several classes of space groups, lattices, point groups, or crystals...
is cubic and its refractive index
Refractive index
In optics the refractive index or index of refraction of a substance or medium is a measure of the speed of light in that medium. It is expressed as a ratio of the speed of light in vacuum relative to that in the considered medium....
(2.41—as measured by sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
light, 589.3 nm) is nearly identical to that of diamond, but the dispersion
Dispersion (optics)
In optics, dispersion is the phenomenon in which the phase velocity of a wave depends on its frequency, or alternatively when the group velocity depends on the frequency.Media having such a property are termed dispersive media...
(the optical property responsible for the "fire" of the cut stones) of strontium titanate is over four times higher, at 0.19 (B–G interval). This results in an excess of fire when compared to diamond.
Synthetics are usually transparent and colourless, but can be doped
Dopant
A dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
with certain rare earth
Rare earth element
As defined by IUPAC, rare earth elements or rare earth metals are a set of seventeen chemical elements in the periodic table, specifically the fifteen lanthanides plus scandium and yttrium...
or transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s to give reds, yellows, browns, and blues. Natural tausonite is usually translucent to opaque, in shades of reddish brown, dark red, or grey. Both have an adamantine (diamond-like) lustre
Lustre (mineralogy)
Lustre is a description of the way light interacts with the surface of a crystal, rock, or mineral. The word lustre traces its origins back to the Latin word lux, meaning "light", and generally implies radiance, gloss, or brilliance....
. Strontium titanate is considered extremely brittle with a conchoidal fracture
Conchoidal fracture
Conchoidal fracture describes the way that brittle materials break when they do not follow any natural planes of separation. Materials that break in this way include flint and other fine-grained minerals, as well as most amorphous solids, such as obsidian and other types of glass.Conchoidal...
; natural material is cubic or octahedral in habit
Crystal habit
Crystal habit is an overall description of the visible external shape of a mineral. This description can apply to an individual crystal or an assembly of crystals or aggregates....
and streaks brown. Through a hand-held (direct vision) spectroscope, doped synthetics will exhibit a rich absorption spectrum typical of doped stones. Synthetic material has a melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
of ca. 2080°C (3776°F) and is readily attacked by hydrofluoric acid
Hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
.
The synthetic material has a very large dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
(300) at room temperature and low electric field. It is also used in high-voltage capacitors. Strontium titanate becomes superconducting below 0.35 K and was the first insulator and oxide discovered to be superconductive.
At temperatures lower than 105 K, its cubic structure transforms to tetragonal. It is an excellent substrate for epitaxial growth of high-temperature superconductors and many oxide-based thin film
Thin film
A thin film is a layer of material ranging from fractions of a nanometer to several micrometers in thickness. Electronic semiconductor devices and optical coatings are the main applications benefiting from thin film construction....
s. Its monocrystals can be used as optical windows and high-quality sputter deposition
Sputter deposition
Sputter deposition is a physical vapor deposition method of depositing thin films by sputtering, that is ejecting, material from a "target," that is source, which then deposits onto a "substrate," such as a silicon wafer...
targets.
SrTiO3 is a suitable material for electronics: niobium
Niobium
Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite...
-doped strontium titanate, is electrically conductive. High-quality, epitaxial SrTiO3 layers can also be grown on silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
without forming silicon dioxide
Silicon dioxide
The chemical compound silicon dioxide, also known as silica , is an oxide of silicon with the chemical formula '. It has been known for its hardness since antiquity...
, thereby making SrTiO3 an alternative gate dielectric material. This also enables the integration of other thin film perovskite oxides onto silicon.
Synthesis

Titanate
In chemistry, titanate usually refers to inorganic compounds composed of titanium oxides. In some cases, the term is used more generally for any titanium-containing anion, e.g. [TiCl6]2- and [Ti7]2-. This article focuses on the oxides....
s patent
Patent
A patent is a form of intellectual property. It consists of a set of exclusive rights granted by a sovereign state to an inventor or their assignee for a limited period of time in exchange for the public disclosure of an invention....
ed during the late 1940s and early 1950s; other titanates included barium titanate
Barium titanate
Barium titanate is the inorganic compound with the chemical formula BaTiO3. Barium titanate is a white powder and transparent as larger crystals...
and calcium titanate
Calcium titanate
Perovskite is a calcium titanium oxide mineral species composed of calcium titanate, with the chemical formula CaTiO3.The mineral was discovered in the Ural Mountains of Russia by Gustav Rose in 1839 and is named after Russian mineralogist Lev Perovski .It lends its name to the class of compounds...
. Research was conducted primarily at the National Lead Company (later renamed N. L. Industries, Inc.) in the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
, by Leon Merker and Langtry E. Lynd. Merker and Lynd first patented the growth process on February 10, 1953; a number of refinements were subsequently patented over the next four years, such as modifications to the feed powder and additions of colouring dopants.
A modification to the basic Verneuil process
Verneuil process
The Verneuil process, also called flame fusion, was the first commercially successful method of manufacturing synthetic gemstones, developed in 1902 by the French chemist Auguste Verneuil. It is primarily used to produce the ruby and sapphire varieties of corundum, as well as the diamond simulants...
(also known as flame-fusion) is the favoured method of growth. An inverted oxy-hydrogen blowpipe
Blowpipe
Blowpipe can refer to:*The Blowpipe missile*Blowgun, a weapon*Blowpipe *Blowpipe , several Transformers characters....
is used, with feed powder mixed with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
carefully fed through the blowpipe in the typical fashion, but with the addition of a third pipe to deliver oxygen—creating a tricone burner. The extra oxygen is required for successful formation of strontium titanate, which would otherwise fail to oxidize completely due to the titanium component. The ratio is ca. 1.5 volumes of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
for each volume of oxygen. The highly purified feed powder is derived by first producing titanyl double oxalate salt
Salt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
(SrTiO(C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
2O4)2·2H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
) by reacting strontium chloride
Strontium chloride
Strontium chloride is a salt of strontium and chloride. It is a typical salt, forming neutral aqueous solutions. Like all compounds of Sr, this salt emits a bright red colour in a flame; in fact is used as a source of redness in fireworks...
(SrCl
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
2) and oxalic acid
Oxalic acid
Oxalic acid is an organic compound with the formula H2C2O4. This colourless solid is a dicarboxylic acid. In terms of acid strength, it is about 3,000 times stronger than acetic acid. Oxalic acid is a reducing agent and its conjugate base, known as oxalate , is a chelating agent for metal cations...
((COOH
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
)2.2H2O) with titanium tetrachloride
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
(TiCl4). The salt is washed to completely eliminate chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
, heated to 1000°C in order to produce a free-flowing granular
powder of the required composition, and is then ground and sieved to ensure all particles are between 0.2–0.5 micrometre
Micrometre
A micrometer , is by definition 1×10-6 of a meter .In plain English, it means one-millionth of a meter . Its unit symbol in the International System of Units is μm...
s in size.
The feed powder falls through the oxyhydrogen flame, melts, and lands on a rotating and slowly descending pedestal below. The height of the pedestal is constantly adjusted to keep its top at the optimal position below the flame, and over a number of hours the molten powder cools and crystallises to form a single pedunculated pear or boule
Boule (crystal)
A boule is a single-crystal ingot produced by synthetic means. A boule of silicon is the starting material for most of the integrated circuits used today....
crystal. This boule is usually no larger than 2.5 centimetres in diameter and 10 centimetres long; it is an opaque black to begin with, requiring further annealing
Annealing (metallurgy)
Annealing, in metallurgy and materials science, is a heat treatment wherein a material is altered, causing changes in its properties such as strength and hardness. It is a process that produces conditions by heating to above the recrystallization temperature, maintaining a suitable temperature, and...
in an oxidizing atmosphere in order to make the crystal colourless and to relieve strain
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some stress which raises its internal energy in comparison to a strain-free reference compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional...
. This is done at over 1000°C for 12 hours.
Use as a diamond simulant
Its cubic structure and high dispersion once made synthetic strontium titanate a prime candidate for simulating diamond. Beginning ca. 1955, large quantities of strontium titanate were manufactured for this sole purpose. Strontium titanate was in competition with synthetic rutileRutile
Rutile is a mineral composed primarily of titanium dioxide, TiO2.Rutile is the most common natural form of TiO2. Two rarer polymorphs of TiO2 are known:...
("titania") at the time, and had the advantage of lacking the unfortunate yellow tinge and strong birefringence
Birefringence
Birefringence, or double refraction, is the decomposition of a ray of light into two rays when it passes through certain anisotropic materials, such as crystals of calcite or boron nitride. The effect was first described by the Danish scientist Rasmus Bartholin in 1669, who saw it in calcite...
inherent to the latter material. While it was softer, it was significantly closer to diamond in likeness. Eventually, however, both would fall into disuse, being eclipsed by the creation of "better" simulants: first by yttrium aluminium garnet
Yttrium aluminium garnet
Yttrium aluminium garnet is a synthetic crystalline material of the garnet group. It is also one of three phases of the yttria-aluminium composite, the other two being yttrium aluminium monoclinic and yttrium aluminium perovskite . YAG is commonly used as a host material in various solid-state...
(YAG) and followed shortly after by gadolinium gallium garnet
Gadolinium gallium garnet
Gadolinium Gallium Garnet is a synthetic crystalline material of the garnet group, with good mechanical, thermal, and optical properties. It is typically colorless. It has cubic lattice, density 7.08 g/cm³ and Mohs hardness is variously noted as 6.5 and 7.5...
(GGG); and finally by the (to date) ultimate simulant in terms of diamond-likeness and cost-effectiveness, cubic zirconia
Cubic zirconia
Cubic zirconia is the cubic crystalline form of zirconium dioxide . The synthesized material is hard, optically flawless and usually colorless, but may be made in a variety of different colors. It should not be confused with zircon, which is a zirconium silicate...
.
Despite being outmoded, strontium titanate is still manufactured and periodically encountered in jewellery. It is one of the most costly of diamond simulants, and due to its rarity collectors may pay a premium for large i.e. >2 carat (400 mg) specimens. As a diamond simulant, strontium titanate is most deceptive when mingled with melée i.e. <0.20 carat (40 mg) stones and when it is used as the base material for a composite or doublet stone (with, e.g., synthetic corundum
Corundum
Corundum is a crystalline form of aluminium oxide with traces of iron, titanium and chromium. It is a rock-forming mineral. It is one of the naturally clear transparent materials, but can have different colors when impurities are present. Transparent specimens are used as gems, called ruby if red...
as the crown or top of the stone). Under the microscope
Microscope
A microscope is an instrument used to see objects that are too small for the naked eye. The science of investigating small objects using such an instrument is called microscopy...
, gemmologist
Gemology
Gemology or gemmology is the science dealing with natural and artificial gems and gemstones. It is considered a geoscience and a branch of mineralogy...
s distinguish strontium titanate from diamond by the former's softness—manifested by surface abrasions—and excess dispersion (to the trained eye), and occasional gas bubbles which are remnants of synthesis. Doublets can be detected by a join line at the girdle ("waist" of the stone) and flattened air bubbles or glue visible within the stone at the point of bonding.

